Our Team
Julien PIRRELLO
MC - INP-ENSAT
julien.pirrello@toulouse-inp.fr
Mondher BOUZAYEN
Professor - INP-ENSAT
mondher.bouzayen@toulouse-inp.fr

Anne BERNADAC
MC - INP-ENSAT
anne.bernadac@toulouse-inp.fr
Christian CHERVIN
Professor - INP-ENSAT
christian.chervin@toulouse-inp.fr

Anis Djari
IE - INP-ENSAT
anis.djari@toulouse-inp.fr

Elie Maza
MC - INP-ENSAT
elie.maza@toulouse-inp.fr
Farid Regad
MC - INP-ENSAT
farid.regad@toulouse-inp.fr
Dominique Saint-Martin
T - INP-ENSAT
dominique.saint-martin@toulouse-inp.fr

Lydie Tessarotto
T - INP-ENSAT
lydie.tessarotto@toulouse-inp.fr
Benoit Van der Rest
MC - INP-ENSAT
benoit.van-der-rest@toulouse-inp.fr

Alice Diot
PhD
alice.diot@toulouse-inp.fr
Olivia Di Valentin
PhD

Amel Djouhri
T CDD
amel.djouhri@toulouse-inp.fr

Yushuo Gao
PhD
yushuo.gao@toulouse-inp.fr

Léa Ghalayini
T CDD
lea.ghalayini@toulouse-inp.fr
Thibault Gillet
AI CDD
thibault.gillet@toulouse-inp.fr
Guillaume Madignier
IE CDD
guillaume.madignier@toulouse-inp.fr
Jing Wang
PhD
jing.wang@toulouse-inp.fr

Shiyu Ying
PhD
shiyu.ying@toulouse-inp.fr
Former members
The making of fruits comprises three main distinct stages known as fruit set, growth and maturation and in this regard, it offers a unique example of a series of developmental shifts tightly regulated by intricate signaling pathways that remain only partially understood. Building on the last period achievements, the GBF research project deals with the deciphering of the factors and mechanisms underlying the flower-to-fruit transition and the transition to ripening of flesh fruits using the tomato as a main model species. In the last period, GBF is also addressing the process of inflorescence meristem differentiation. GBF team addresses the link between hormone signaling and epigenetic regulation in controlling gene expression reprogramming during all these developmental processes. The group also investigates the genetic basis of the differentiation of fleshy tissues through the characterization of master regulators of fruit tissue texture, a major trait impacting quality and post-harvest behaviour. The elucidation of the processes of fruit setting and flower formation is fundamental to our understanding of reproductive organs biology and is anticipated to provide the basis for designing new breeding strategies to improve crop yield and fruit quality in the face of climate change. Likewise, fruit ripening is of prime importance as most sensory and nutritional quality traits take place during this ultimate phase of fruit development.
On the other hand, the GBF group continue to make a substantial contribution to the generation of generic resources and tools on the tomato model species through developing functional genomics platforms accessible to the scientific community. Building on the expertise gained on the tomato model, the group invested recently in the generation of genomic and transcriptomic resources on grape as a new fleshy fruit species.
It is also worth mentioning that in support to its scientific programs, GBF is involved in major EU initiatives and is currently coordinating a COST European network dealing with the role of oxygen sensing in fleshy fruit ripening.
- Onset of fruit ripening
- Fruit texture and differentiation of fleshy tissues
- Molecular and genetic factors controlling inflorescence differentiation and fruit set
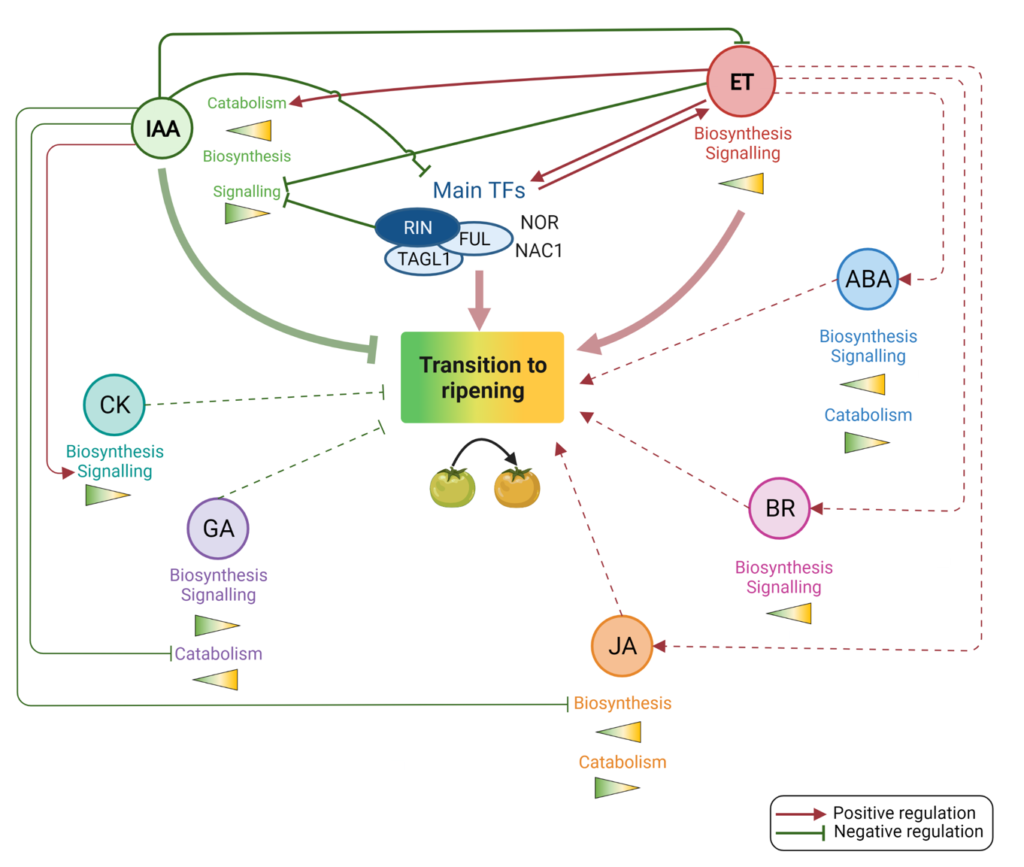
Fruit development consists of a series of developmental transitions, among which fruit ripening corresponds to the ultimate step, associated with seed maturation and dispersal. Tomato fruit is known to undergo climacteric ripening that is characterized by a sharp increase in respiration, the so-called “climacteric crisis”, and a rise in autocatalytic ethylene production. Ethylene is the main hormone controlling climacteric fruit ripening. While studies on ethylene are numerous, the mechanisms underlying the developmental transition leading to the initiation of the ripening process remains elusive, although it has been postulated that an active hormone interplay is involved in driving this process, as it is the case for fruit set (Chirinos et al 2022, Plant Phys.; An et al. 2020, Plant Science). In despite of the tremendous progress made in understanding the role of ethylene in controlling climacteric fruit ripening the putative link between the rise in respiration and the increase of ethylene production remains totally obscure. The elucidation made recently in the model plant Arabidopsis of the role of Ethylene Response Factors in oxygen sensing opened new prospects towards addressing the role of the increase in respiration during the climacteric ripening.
Ethylene receptors are encoded in the tomato by seven genes, named SlETR1 to SlETR7. Performing targeted proteomics analysis of ethylene tomato receptors, we recently showed that ripening inhibition in the tomato may be associated with the increase of the ETR3 dominant mutated receptor (Chen et al. 2019, Frontiers Plant Sci.). Our data also revealed that ethylene sensing is critical for tomato pollen tube development, controlling at least cell wall metabolism and calcium signaling (Althiab-Almasaud et al., 2021a, Revue?).
The linear ethylene production and signaling cascade ultimately leads to the activation of members of the large Ethylene Response Factor (ERF) transcription factor family. Although it has been often suggested that that ERFs are at the internode of different hormonal signaling pathways, data regarding the role of different hormones during the onset of ripening are still scarce. Using transcriptomics and hormone profiling approaches, we recently showed that the transition to ripening is initiated in the tissues filling the locular cavities and that the transcriptomic reprogramming underlying this developmental shift requires a multi-hormonal control and a reformed auxin homeostasis, and most likely other hormones seems to be involved in this transitioning (Chirinos et al., 2022, Plant Phys.).
Softening and internal fruit structure are traits of prime importance for tomato sensory quality, postharvest behavior and overall commercial value. It is also a major criterion in determining whether the crop is better suited for fresh consumption or the processing industry. The dynamics of fleshy fruit tissue texture is complex, and softness has always been addressed as a ripening associated process. Notably, so far, all the strategies aiming at improving fruit texture and postharvest shelf-life consisted in attenuating the ripening process which inevitably leads to losses in sensory qualities resulting from incomplete ripening. It is noteworthy that “All-flesh” tomato cultivars are devoid of locular gel and exhibit enhanced firmness and improved postharvest storage. Our group aims to understand the process determining firmness and texture. Although the deciphering of the components underlying the softening process has been mostly addressed by focusing on late stages of fruit development, our data are consistent with the notion that a large component of texture and firmness of ripe fruit is determined at early pre-ripening stages, concomitant with the initiation of inner tissues differentiation. Using reversed genetics, ChIPseq, transcritptomic and microscopy techniques we highlighted the role of MBP3, a class-D MADS-Box, in locular gel formation by controlling cell cycle and cell expansion genes, indicating that important components of fruit softening are determined at early pre-ripening stages. Our findings brings a paradigm shift of thinking and provides new opportunities for breeding strategies aiming to design tomato varieties, and possibly other fleshy fruits, with specific texture properties (Huang et al. 2021, Nature Com.). Such new varieties will be better suited for tomato processing and the extended shelf life of the fresh fruits will contribute to reducing wastage.

In flowering plants, differentiation of reproductive organs and successful flower fertilization are among the main determinants of crop yield, and in most crop species, yield relies primarily on the number of inflorescences and of flowers per inflorescence. Fruit set is naturally triggered by flower pollination and represents another important developmental process that impacts crop yield. Using the tomato as model species, we address the mechanisms of fruit set and inflorescence differentiation as two major steps determining fruit yield.
The transition from flower to fruit, the so-called fruit set, is an important determinant of crop yield and in the face of global warming and fast-growing world population, maintaining yield stability is becoming a major challenge. We investigate the epigenetic and transcriptomic reprogramming underlying pollination-dependent and auxin-induced flower-to-fruit transitions in the tomato (Solanum lycopersicum L.) using combined genome-wide transcriptomic profiling, global ChIP-sequencing and whole genomic DNA bisulfite sequencing (WGBS). Our data demonstrate reprogramming of genes involved in processes instrumental to fruit set correlated with their H3K9ac or H3K4me3 marking status but not with changes in cytosine methylation, indicating that histone post translational modifications rather than DNA methylation are associated with the remodeling of the epigenetic landscape underpinning the flower-to-fruit transition. Given the prominent role previously assigned to DNA methylation in reprogramming key genes of the transition to ripening, the outcome of the present study supports the idea that the two main developmental transitions in fleshy fruit and the underlying transcriptomic reprogramming are associated with different modes of epigenetic regulations (Hu et al. 2020, New Phytologist).
Understanding the mechanisms underlying differentiation of inflorescence and flower meristems is essential towards enlarging our knowledge of reproductive organ formation and to open new prospects for improving yield traits. Using CRISPR/Cas9 knock-out strategy, our recent study shows that SlDOF9 is a new modulator of floral differentiation in tomato. Our findings add a new actor to the complex mechanisms underlying reproductive organ differentiation in flowering plants and provide leads towards addressing the diversity of factors controlling the transition to reproductive organs (Hu et al 2022, Nature Plants).

Publications
1: Hu G, Wang K, Huang B, Mila I, Frasse P, Maza E, Djari A, Hernould M, Zouine M, Li Z, Bouzayen M. The auxin-responsive transcription factor SlDOF9 regulates inflorescence and flower development in tomato. Nat Plants. 2022 Apr;8(4):419-433. doi: 10.1038/s41477-022-01121-1. Epub 2022 Apr 14. PMID: 35422080.
2: Wu CJ, Shan W, Liu XC, Zhu LS, Wei W, Yang YY, Guo YF, Bouzayen M, Chen JY, Lu WJ, Kuang JF. Phosphorylation of transcription factor bZIP21 by MAP kinase MPK6-3 enhances banana fruit ripening. Plant Physiol. 2022 Mar 4;188(3):1665-1685. doi: 10.1093/plphys/kiab539. PMID: 34792564; PMCID: PMC8896643.
3: Huang B, Hu G, Wang K, Frasse P, Maza E, Djari A, Deng W, Pirrello J, Burlat V, Pons C, Granell A, Li Z, van der Rest B, Bouzayen M. Interaction of two MADS- box genes leads to growth phenotype divergence of all-flesh type of tomatoes. Nat Commun. 2021 Nov 25;12(1):6892. doi: 10.1038/s41467-021-27117-7. PMID: 34824241; PMCID: PMC8616914.
4: Bineau E, Diouf I, Carretero Y, Duboscq R, Bitton F, Djari A, Zouine M, Causse M. Genetic diversity of tomato response to heat stress at the QTL and transcriptome levels. Plant J. 2021 Aug;107(4):1213-1227. doi: 10.1111/tpj.15379. Epub 2021 Aug 24. PMID: 34160103.
5: Chirinos X, Ying S, Rodrigues MA, Maza E, Djari A, Hu G, Liu M, Purgatto E, Fournier S, Regad F, Bouzayen M, Pirrello J. Transition to ripening in tomato requires hormone-controlled genetic reprogramming initiated in gel tissue. Plant Physiol. 2023 Jan 2;191(1):610-625. doi: 10.1093/plphys/kiac464. PMID: 36200876; PMCID: PMC9806557.
6: Althiab-Almasaud R, Chen Y, Maza E, Djari A, Frasse P, Mollet JC, Mazars C, Jamet E, Chervin C. Ethylene signaling modulates tomato pollen tube growth through modifications of cell wall remodeling and calcium gradient. Plant J. 2021 Aug;107(3):893-908. doi: 10.1111/tpj.15353. Epub 2021 Jun 20. PMID: 34036648.
7: Hu G, Huang B, Wang K, Frasse P, Maza E, Djari A, Benhamed M, Gallusci P, Li Z, Zouine M, Bouzayen M. Histone posttranslational modifications rather than DNA methylation underlie gene reprogramming in pollination-dependent and pollination-independent fruit set in tomato. New Phytol. 2021 Jan;229(2):902-919. doi: 10.1111/nph.16902. Epub 2020 Oct 27. PMID: 32875585; PMCID: PMC7821339.
1: Egea I, Barsan C, Bian W, Purgatto E, Latché A, Chervin C, Bouzayen M, Pech
- Chromoplast differentiation: current status and perspectives. Plant Cell
Physiol. 2010 Oct;51(10):1601-11. doi: 10.1093/pcp/pcq136. Epub 2010 Aug 27.
PMID: 20801922.
2: Van Der Straeten D, Kanellis A, Kalaitzis P, Bouzayen M, Chang C, Mattoo A,
Zhang JS. Editorial: Ethylene Biology and Beyond: Novel Insights in the Ethylene
Pathway and Its Interactions. Front Plant Sci. 2020 Mar 12;11:248. doi:
10.3389/fpls.2020.00248. PMID: 32226435; PMCID: PMC7080854.
3: Liu M, Pirrello J, Chervin C, Roustan JP, Bouzayen M. Ethylene Control of
Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation.
Plant Physiol. 2015 Dec;169(4):2380-90. doi: 10.1104/pp.15.01361. Epub 2015 Oct
- PMID: 26511917; PMCID: PMC4677914.
4: Petek M, Zagorščak M, Ramšak Ž, Sanders S, Tomaž Š, Tseng E, Zouine M, Coll
A, Gruden K. Cultivar-specific transcriptome and pan-transcriptome
reconstruction of tetraploid potato. Sci Data. 2020 Jul 24;7(1):249. doi:
10.1038/s41597-020-00581-4. PMID: 32709858; PMCID: PMC7382494.
5: Liu R, How-Kit A, Stammitti L, Teyssier E, Rolin D, Mortain-Bertrand A, Halle
S, Liu M, Kong J, Wu C, Degraeve-Guibault C, Chapman NH, Maucourt M, Hodgman TC,
Tost J, Bouzayen M, Hong Y, Seymour GB, Giovannoni JJ, Gallusci P. A DEMETER-
like DNA demethylase governs tomato fruit ripening. Proc Natl Acad Sci U S A.
2015 Aug 25;112(34):10804-9. doi: 10.1073/pnas.1503362112. Epub 2015 Aug 10.
PMID: 26261318; PMCID: PMC4553810.
6: Pons S, Fournier S, Chervin C, Bécard G, Rochange S, Frei Dit Frey N, Puech
Pagès V. Phytohormone production by the arbuscular mycorrhizal fungus
Rhizophagus irregularis. PLoS One. 2020 Oct 16;15(10):e0240886. doi:
10.1371/journal.pone.0240886. PMID: 33064769; PMCID: PMC7567356.
7: Su LY, Audran C, Bouzayen M, Roustan JP, Chervin C. The Aux/IAA, Sl-IAA17
regulates quality parameters over tomato fruit development. Plant Signal Behav.
2015;10(11):e1071001. doi: 10.1080/15592324.2015.1071001. Erratum in: doi:
10.1093/pcp/pcu124. PMID: 26317283; PMCID: PMC4883969.
8: Barsan C, Kuntz M, Pech JC. Isolation of Chromoplasts and Suborganellar
Compartments from Tomato and Bell Pepper Fruit. Methods Mol Biol.
2017;1511:61-71. doi: 10.1007/978-1-4939-6533-5_5. PMID: 27730602.
9: Etemadi M, Gutjahr C, Couzigou JM, Zouine M, Lauressergues D, Timmers A,
Audran C, Bouzayen M, Bécard G, Combier JP. Auxin perception is required for
arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014
Sep;166(1):281-92. doi: 10.1104/pp.114.246595. Epub 2014 Aug 5. PMID: 25096975;
PMCID: PMC4149713.
10: Guillotin B, Etemadi M, Audran C, Bouzayen M, Bécard G, Combier JP. Sl-IAA27
regulates strigolactone biosynthesis and mycorrhization in tomato (var.
MicroTom). New Phytol. 2017 Feb;213(3):1124-1132. doi: 10.1111/nph.14246. Epub
2016 Oct 17. PMID: 27748948.
11: Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z, Roustan JP, Bouzayen
M, Giuliano G, Chervin C. Carotenoid accumulation during tomato fruit ripening
is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015 May 8;15:114.
doi: 10.1186/s12870-015-0495-4. PMID: 25953041; PMCID: PMC4424491.
12: Jian W, Cao H, Yuan S, Liu Y, Lu J, Lu W, Li N, Wang J, Zou J, Tang N, Xu C,
Cheng Y, Gao Y, Xi W, Bouzayen M, Li Z. SlMYB75, an MYB-type transcription
factor, promotes anthocyanin accumulation and enhances volatile aroma production
in tomato fruits. Hortic Res. 2019 Feb 1;6:22. doi: 10.1038/s41438-018-0098-y.
PMID: 30729012; PMCID: PMC6355774.
13: El-Sharkawy I, Sherif SM, Jones B, Mila I, Kumar PP, Bouzayen M, Jayasankar
- TIR1-like auxin-receptors are involved in the regulation of plum fruit
development. J Exp Bot. 2014 Oct;65(18):5205-15. doi: 10.1093/jxb/eru279. Epub
2014 Jul 4. PMID: 24996652; PMCID: PMC4157706.
14: Dai Y, Hu G, Dupas A, Medina L, Blandels N, Clemente HS, Ladouce N, Badawi
M, Hernandez-Raquet G, Mounet F, Grima-Pettenati J, Cassan-Wang H. Implementing
the CRISPR/Cas9 Technology in <i>Eucalyptus</i> Hairy Roots Using Wood-Related
Genes. Int J Mol Sci. 2020 May 12;21(10):3408. doi: 10.3390/ijms21103408. PMID:
32408486; PMCID: PMC7279396.
15: Liu M, Chen Y, Chen Y, Shin JH, Mila I, Audran C, Zouine M, Pirrello J,
Bouzayen M. The tomato Ethylene Response Factor Sl-ERF.B3 integrates ethylene
and auxin signaling via direct regulation of Sl-Aux/IAA27. New Phytol. 2018
Jul;219(2):631-640. doi: 10.1111/nph.15165. Epub 2018 Apr 27. PMID: 29701899.
16: Shan W, Kuang JF, Wei W, Fan ZQ, Deng W, Li ZG, Bouzayen M, Pirrello J, Lu
WJ, Chen JY. MaXB3 Modulates MaNAC2, MaACS1, and MaACO1 Stability to Repress
Ethylene Biosynthesis during Banana Fruit Ripening. Plant Physiol. 2020
Oct;184(2):1153-1171. doi: 10.1104/pp.20.00313. Epub 2020 Jul 21. PMID:
32694134; PMCID: PMC7536691.
17: Deng W, Yang Y, Ren Z, Audran-Delalande C, Mila I, Wang X, Song H, Hu Y,
Bouzayen M, Li Z. The tomato SlIAA15 is involved in trichome formation and
axillary shoot development. New Phytol. 2012 Apr;194(2):379-390. doi:
10.1111/j.1469-8137.2012.04053.x. Epub 2012 Mar 12. PMID: 22409484.
18: Makhloufi E, Yousfi FE, Pirrello J, Bernadac A, Ghorbel A, Bouzayen M.
TdERF1, an ethylene response factor associated with dehydration responses in
durum wheat (Triticum turgidum L. subsp. durum). Plant Signal Behav.
2015;10(10):e1065366. doi: 10.1080/15592324.2015.1065366. Epub 2015 Sep 4.
Erratum in: doi: 10.1093/jxb/eru352. PMID: 26338450; PMCID: PMC4883918.
19: Barsan C, Sanchez-Bel P, Rombaldi C, Egea I, Rossignol M, Kuntz M, Zouine M,
Latché A, Bouzayen M, Pech JC. Characteristics of the tomato chromoplast
revealed by proteomic analysis. J Exp Bot. 2010 May;61(9):2413-31. doi:
10.1093/jxb/erq070. Epub 2010 Apr 2. PMID: 20363867.
20: Chaabouni S, Pirrello J, Liu M, El-Sharkawy I, Roustan JP, Bouzayen M.
Identification and functional characterization of two HOOKLESS genes in Tomato
(Solanum lycopersicum). J Plant Physiol. 2016 Aug 1;200:76-81. doi:
10.1016/j.jplph.2016.05.017. Epub 2016 Jun 9. PMID: 27343715.
21: Bakshi A, Piya S, Fernandez JC, Chervin C, Hewezi T, Binder BM. Ethylene
Receptors Signal via a Noncanonical Pathway to Regulate Abscisic Acid Responses.
Plant Physiol. 2018 Jan;176(1):910-929. doi: 10.1104/pp.17.01321. Epub 2017 Nov
- PMID: 29158332; PMCID: PMC5761792.
22: Djemal R, Mila I, Bouzayen M, Pirrello J, Khoudi H. Molecular cloning and
characterization of novel WIN1/SHN1 ethylene responsive transcription factor
HvSHN1 in barley (Hordeum vulgare L.). J Plant Physiol. 2018 Sep;228:39-46. doi:
10.1016/j.jplph.2018.04.019. Epub 2018 May 28. PMID: 29852333.
23: Yu H, Soler M, Mila I, San Clemente H, Savelli B, Dunand C, Paiva JA, Myburg
AA, Bouzayen M, Grima-Pettenati J, Cassan-Wang H. Genome-wide characterization
and expression profiling of the AUXIN RESPONSE FACTOR (ARF) gene family in
Eucalyptus grandis. PLoS One. 2014 Sep 30;9(9):e108906. doi:
10.1371/journal.pone.0108906. PMID: 25269088; PMCID: PMC4182523.
24: Su L, Bassa C, Audran C, Mila I, Cheniclet C, Chevalier C, Bouzayen M,
Roustan JP, Chervin C. The auxin Sl-IAA17 transcriptional repressor controls
fruit size via the regulation of endoreduplication-related cell expansion. Plant
Cell Physiol. 2014 Nov;55(11):1969-76. doi: 10.1093/pcp/pcu124. Epub 2014 Sep
- PMID: 25231966.
25: Zouine M, Fu Y, Chateigner-Boutin AL, Mila I, Frasse P, Wang H, Audran C,
Roustan JP, Bouzayen M. Characterization of the tomato ARF gene family uncovers
a multi-levels post-transcriptional regulation including alternative splicing.
PLoS One. 2014 Jan 10;9(1):e84203. doi: 10.1371/journal.pone.0084203. PMID:
24427281; PMCID: PMC3888382.
26: Hu G, Huang B, Wang K, Frasse P, Maza E, Djari A, Benhamed M, Gallusci P, Li
Z, Zouine M, Bouzayen M. Histone posttranslational modifications rather than DNA
methylation underlie gene reprogramming in pollination-dependent and
pollination-independent fruit set in tomato. New Phytol. 2021
Jan;229(2):902-919. doi: 10.1111/nph.16902. Epub 2020 Oct 27. PMID: 32875585;
PMCID: PMC7821339.
27: Audran-Delalande C, Bassa C, Mila I, Regad F, Zouine M, Bouzayen M. Genome-
wide identification, functional analysis and expression profiling of the Aux/IAA
gene family in tomato. Plant Cell Physiol. 2012 Apr;53(4):659-72. doi:
10.1093/pcp/pcs022. Epub 2012 Feb 24. PMID: 22368074.
28: Hao Y, Hu G, Breitel D, Liu M, Mila I, Frasse P, Fu Y, Aharoni A, Bouzayen
M, Zouine M. Auxin Response Factor SlARF2 Is an Essential Component of the
Regulatory Mechanism Controlling Fruit Ripening in Tomato. PLoS Genet. 2015 Dec
30;11(12):e1005649. doi: 10.1371/journal.pgen.1005649. PMID: 26716451; PMCID:
PMC4696797.
29: Breitel DA, Chappell-Maor L, Meir S, Panizel I, Puig CP, Hao Y, Yifhar T,
Yasuor H, Zouine M, Bouzayen M, Granell Richart A, Rogachev I, Aharoni A. AUXIN
RESPONSE FACTOR 2 Intersects Hormonal Signals in the Regulation of Tomato Fruit
Ripening. PLoS Genet. 2016 Mar 9;12(3):e1005903. doi:
10.1371/journal.pgen.1005903. PMID: 26959229; PMCID: PMC4784954.
30: Yu H, Soler M, San Clemente H, Mila I, Paiva JA, Myburg AA, Bouzayen M,
Grima-Pettenati J, Cassan-Wang H. Comprehensive genome-wide analysis of the
Aux/IAA gene family in Eucalyptus: evidence for the role of EgrIAA4 in wood
formation. Plant Cell Physiol. 2015 Apr;56(4):700-14. doi: 10.1093/pcp/pcu215.
Epub 2015 Jan 9. PMID: 25577568.
31: Moummou H, Tonfack LB, Chervin C, Benichou M, Youmbi E, Ginies C, Latché A,
Pech JC, van der Rest B. Functional characterization of SlscADH1, a fruit-
ripening-associated short-chain alcohol dehydrogenase of tomato. J Plant
Physiol. 2012 Oct 15;169(15):1435-44. doi: 10.1016/j.jplph.2012.06.007. Epub
2012 Jul 20. PMID: 22818888.
32: Salma C, Alain L, Claude PJ, Mondher B. Tomato Aux/IAA3 and HOOKLESS are
important actors of the interplay between auxin and ethylene during apical hook
formation. Plant Signal Behav. 2009 Jun;4(6):559-60. doi: 10.1093/jxb/erp009.
Epub 2009 Jun 17. PMID: 19816148; PMCID: PMC2688313.
33: Moummou H, Kallberg Y, Tonfack LB, Persson B, van der Rest B. The plant
short-chain dehydrogenase (SDR) superfamily: genome-wide inventory and
diversification patterns. BMC Plant Biol. 2012 Nov 20;12:219. doi:
10.1186/1471-2229-12-219. PMID: 23167570; PMCID: PMC3541173.
34: Tohge T, Scossa F, Wendenburg R, Frasse P, Balbo I, Watanabe M, Alseekh S,
Jadhav SS, Delfin JC, Lohse M, Giavalisco P, Usadel B, Zhang Y, Luo J, Bouzayen
M, Fernie AR. Exploiting Natural Variation in Tomato to Define Pathway Structure
and Metabolic Regulation of Fruit Polyphenolics in the Lycopersicum Complex. Mol
Plant. 2020 Jul 6;13(7):1027-1046. doi: 10.1016/j.molp.2020.04.004. Epub 2020
Apr 16. PMID: 32305499.
35: Makhloufi E, Yousfi FE, Marande W, Mila I, Hanana M, Bergès H, Mzid R,
Bouzayen M. Isolation and molecular characterization of ERF1, an ethylene
response factor gene from durum wheat (Triticum turgidum L. subsp. durum),
potentially involved in salt-stress responses. J Exp Bot. 2014
Dec;65(22):6359-71. doi: 10.1093/jxb/eru352. Epub 2014 Sep 9. PMID: 25205575.
36: Yousfi FE, Makhloufi E, Marande W, Ghorbel AW, Bouzayen M, Bergès H.
Comparative Analysis of <i>WRKY</i> Genes Potentially Involved in Salt Stress
Responses in <i>Triticum turgidum L</i>. ssp. <i>durum</i>. Front Plant Sci.
2017 Jan 31;7:2034. doi: 10.3389/fpls.2016.02034. PMID: 28197152; PMCID:
PMC5281569.
37: Huang B, Routaboul JM, Liu M, Deng W, Maza E, Mila I, Hu G, Zouine M, Frasse
P, Vrebalov JT, Giovannoni JJ, Li Z, van der Rest B, Bouzayen M. Overexpression
of the class D MADS-box gene Sl-AGL11 impacts fleshy tissue differentiation and
structure in tomato fruits. J Exp Bot. 2017 Oct 13;68(17):4869-4884. doi:
10.1093/jxb/erx303. PMID: 28992179.
38: Chaabouni S, Jones B, Delalande C, Wang H, Li Z, Mila I, Frasse P, Latché A,
Pech JC, Bouzayen M. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and
ethylene signalling involved in differential growth. J Exp Bot.
2009;60(4):1349-62. doi: 10.1093/jxb/erp009. Epub 2009 Feb 12. PMID: 19213814;
PMCID: PMC2657550.
39: Liu M, Diretto G, Pirrello J, Roustan JP, Li Z, Giuliano G, Regad F,
Bouzayen M. The chimeric repressor version of an Ethylene Response Factor (ERF)
family member, Sl-ERF.B3, shows contrasting effects on tomato fruit ripening.
New Phytol. 2014 Jul;203(1):206-18. doi: 10.1111/nph.12771. Epub 2014 Mar 20.
PMID: 24645853.
40: Liu M, Gomes BL, Mila I, Purgatto E, Peres LE, Frasse P, Maza E, Zouine M,
Roustan JP, Bouzayen M, Pirrello J. Comprehensive Profiling of Ethylene Response
Factor Expression Identifies Ripening-Associated ERF Genes and Their Link to Key
Regulators of Fruit Ripening in Tomato. Plant Physiol. 2016 Mar;170(3):1732-44.
doi: 10.1104/pp.15.01859. Epub 2016 Jan 6. PMID: 26739234; PMCID: PMC4775140.
41: Liu M, Pirrello J, Kesari R, Mila I, Roustan JP, Li Z, Latché A, Pech JC,
Bouzayen M, Regad F. A dominant repressor version of the tomato Sl-ERF.B3 gene
confers ethylene hypersensitivity via feedback regulation of ethylene signaling
and response components. Plant J. 2013 Nov;76(3):406-19. doi: 10.1111/tpj.12305.
Epub 2013 Oct 3. PMID: 23931552.
42: Zouine M, Maza E, Djari A, Lauvernier M, Frasse P, Smouni A, Pirrello J,
Bouzayen M. TomExpress, a unified tomato RNA-Seq platform for visualization of
expression data, clustering and correlation networks. Plant J. 2017
Nov;92(4):727-735. doi: 10.1111/tpj.13711. Epub 2017 Oct 25. PMID: 28873253.
43: Louveau T, Leitao C, Green S, Hamiaux C, van der Rest B, Dechy-Cabaret O,
Atkinson RG, Chervin C. Predicting the substrate specificity of a
glycosyltransferase implicated in the production of phenolic volatiles in tomato
fruit. FEBS J. 2011 Jan;278(2):390-400. doi: 10.1111/j.1742-4658.2010.07962.x.
Epub 2010 Dec 17. PMID: 21166996.
44: Trapet P, Avoscan L, Klinguer A, Pateyron S, Citerne S, Chervin C, Mazurier
S, Lemanceau P, Wendehenne D, Besson-Bard A. The Pseudomonas fluorescens
Siderophore Pyoverdine Weakens Arabidopsis thaliana Defense in Favor of Growth
in Iron-Deficient Conditions. Plant Physiol. 2016 May;171(1):675-93. doi:
10.1104/pp.15.01537. Epub 2016 Mar 8. PMID: 26956666; PMCID: PMC4854674.
45: Sagar M, Chervin C, Mila I, Hao Y, Roustan JP, Benichou M, Gibon Y, Biais B,
Maury P, Latché A, Pech JC, Bouzayen M, Zouine M. SlARF4, an auxin response
factor involved in the control of sugar metabolism during tomato fruit
development. Plant Physiol. 2013 Mar;161(3):1362-74. doi: 10.1104/pp.113.213843.
Epub 2013 Jan 22. PMID: 23341361; PMCID: PMC3585602.
46: Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latché A, Pech
JC, Fernie AR, Bouzayen M. Regulatory features underlying pollination-dependent
and -independent tomato fruit set revealed by transcript and primary metabolite
profiling. Plant Cell. 2009 May;21(5):1428-52. doi: 10.1105/tpc.108.060830. Epub
2009 May 12. PMID: 19435935; PMCID: PMC2700536.
47: Bassa C, Mila I, Bouzayen M, Audran-Delalande C. Phenotypes associated with
down-regulation of Sl-IAA27 support functional diversity among Aux/IAA family
members in tomato. Plant Cell Physiol. 2012 Sep;53(9):1583-95. doi:
10.1093/pcp/pcs101. Epub 2012 Jul 3. PMID: 22764281.
48: Maza E, Frasse P, Senin P, Bouzayen M, Zouine M. Comparison of normalization
methods for differential gene expression analysis in RNA-Seq experiments: A
matter of relative size of studied transcriptomes. Commun Integr Biol. 2013 Nov
1;6(6):e25849. doi: 10.4161/cib.25849. Epub 2013 Jul 30. PMID: 26442135; PMCID:
PMC3918003.
49: Egea I, Bian W, Barsan C, Jauneau A, Pech JC, Latché A, Li Z, Chervin C.
Chloroplast to chromoplast transition in tomato fruit: spectral confocal
microscopy analyses of carotenoids and chlorophylls in isolated plastids and
time-lapse recording on intact live tissue. Ann Bot. 2011 Aug;108(2):291-7. doi:
10.1093/aob/mcr140. PMID: 21788376; PMCID: PMC3143050.
50: Pirrello J, Jaimes-Miranda F, Sanchez-Ballesta MT, Tournier B, Khalil-Ahmad
Q, Regad F, Latché A, Pech JC, Bouzayen M. Sl-ERF2, a tomato ethylene response
factor involved in ethylene response and seed germination. Plant Cell Physiol.
2006 Sep;47(9):1195-205. doi: 10.1093/pcp/pcj084. Epub 2006 Jul 20. PMID:
16857696.
51: Manríquez D, El-Sharkawy I, Flores FB, El-Yahyaoui F, Regad F, Bouzayen M,
Latché A, Pech JC. Two highly divergent alcohol dehydrogenases of melon exhibit
fruit ripening-specific expression and distinct biochemical characteristics.
Plant Mol Biol. 2006 Jul;61(4-5):675-85. doi: 10.1007/s11103-006-0040-9. PMID:
16897483.
52: Ambrosino L, Ruggieri V, Bostan H, Miralto M, Vitulo N, Zouine M, Barone A,
Bouzayen M, Frusciante L, Pezzotti M, Valle G, Chiusano ML. Multilevel
comparative bioinformatics to investigate evolutionary relationships and
specificities in gene annotations: an example for tomato and grapevine. BMC
Bioinformatics. 2018 Nov 30;19(Suppl 15):435. doi: 10.1186/s12859-018-2420-y.
PMID: 30497367; PMCID: PMC6266932.
53: Barsan C, Zouine M, Maza E, Bian W, Egea I, Rossignol M, Bouyssie D,
Pichereaux C, Purgatto E, Bouzayen M, Latché A, Pech JC. Proteomic analysis of
chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled
with disrupted thylakoid biogenesis machinery and elevated energy-production
components. Plant Physiol. 2012 Oct;160(2):708-25. doi: 10.1104/pp.112.203679.
Epub 2012 Aug 20. PMID: 22908117; PMCID: PMC3461550.
54: Chervin C, Tira-Umphon A, Terrier N, Zouine M, Severac D, Roustan JP.
Stimulation of the grape berry expansion by ethylene and effects on related gene
transcripts, over the ripening phase. Physiol Plant. 2008 Nov;134(3):534-46.
doi: 10.1111/j.1399-3054.2008.01158.x. Epub 2008 Jul 31. PMID: 18785902.
55: El-Sharkawy I, Manríquez D, Flores FB, Regad F, Bouzayen M, Latché A, Pech
- Functional characterization of a melon alcohol acyl-transferase gene family
involved in the biosynthesis of ester volatiles. Identification of the crucial
role of a threonine residue for enzyme activity*. Plant Mol Biol. 2005
Sep;59(2):345-62. doi: 10.1007/s11103-005-8884-y. PMID: 16247561.
56: Lucchetta L, Manriquez D, El-Sharkawy I, Flores FB, Sanchez-Bel P, Zouine M,
Ginies C, Bouzayen M, Rombaldi C, Pech JC, Latché A. Biochemical and catalytic
properties of three recombinant alcohol acyltransferases of melon. sulfur-
containing ester formation, regulatory role of CoA-SH in activity, and sequence
elements conferring substrate preference. J Agric Food Chem. 2007 Jun
27;55(13):5213-20. doi: 10.1021/jf070210w. Epub 2007 Jun 2. PMID: 17542607.
lrsv.gestion@univ-tlse3.fr.
Phone
Standard : +33 5.34.32.38.01
Fax : +33 5.34.32.38.02
Find us
24, chemin de Borde-Rouge.BP 42617 Auzeville.
31326, Castanet-Tolosan. FRANCE




