
Christophe ROUX
Responsable Equipe RHIZO, Professeur des Universités
christophe.roux@univ-tlse3.fr

Bernard DUMAS
Directeur de Recherche, CNRS
bernard.dumas@univ-tlse3.fr

Guillaume BECARD
Professeur des Universités
guillaume.becard@univ-tlse3.fr

Jean-Malo COUZIGOU
Chargé de Recherche, CNRS
jean-malo.couzigou@univ-tlse3.fr

Nathalie SEJALON-DELMAS
Maitre de Conférence
nathalie.sejalon-delmas@univ-tlse3.fr

Elodie GAULIN
Maître de Conférence
elodie.gaulin@univ-tlse3.fr

Laurent CAMBORDE
Ingénieur de Recherche CNRS
laurent.camborde@univ-tlse3.fr

Francis CARBONNE
Assistant Ingénieur, CNRS
francis.carbonne@univ-tlse3.fr
Mohamed Zouaoui
Ingénieur de Recherche Université Toulouse 3 - Labcom
mohamed.zouaoui@univ-tlse3.fr

Margot TRINQUIER
Post-Doctorante, CNRS
Margot.Trinquier@inrae.fr
The RHIZO Students
Master, BTS, Licence
Research Topics
In natural environment, plants interact with a wide variety of microbes, including bacteria, fungi and oomycetes. Only 10 years ago, it was supposed that most of these microbes were present at the plant surfaces, a small fraction of them being able to penetrate root tissues. Depending on the resulting effect on plant, such invasive microbes were defined as parasitic, mutualistic and sometimes endophytic when the beneficial or detrimental effect on plant where not easy to characterize. Thanks to the next generation DNA sequencing approaches, it was discovered that plant tissues are not sterile, but host an internal microbiome whose composition is a subset of the external microbial community. This opens a new front of science on the incidence on plant development and immunity of microbial communities outside and inside plant tissues. Particularly, roots are associated with complex soil microbes that impact plant health, growth and productivity. Whereas numerous studies investigate the composition of root associated microbiome – in roots and rhizosphere – less is known on its functioning and its incidence on the life of plants.
The team Microbial Interactions in Rhizosphere and Roots (RHIZO) investigates how microbe-microbe interactions drive the outcome of plant-microbe interactions. For that we rely on our favorite microbial models: Arbuscular Mycorrhizal (AM) fungi (Rhizophagus irregularis), Oomycota (Aphanomyces euteiches and Pythium oligandrum), Actinomycota (Streptomyces sp.) and Rhizobia (Sinorhizobium and Bradyrhizobium).
We consider these microbes not only as plant-interacting organisms, but also as organisms interacting with other microbes in the rhizosphere. By combining approaches of functional and comparative genomics, metagenomics and molecular cell biology, our goal is to study the influence on plant-microbe interactions of microbe-microbe interactions in roots and in the rhizosphere.
The RHIZO team is organized around three mains topics presented below
- Microbial Effectors & Host Targets
- Microbial Key Factors of Symbiotic Efficiency
- Dynamic & Functionality of Rhizosphere Microbiota
Oomycetes Effectors
Oomycetes are filamentous eukaryotic organisms whose close cousins are brown algae and diatoms. Found in both aquatic and terrestrial environments, oomycetes cause a great deal of damage in various ecosystems. Virulent oomycetes produce molecules called effectors, allowing them to counteract host immune defenses and / or disturb the physiology of the host. These effectors, often of a protein nature, interact with cellular components (DNA, RNA, protein …) in order to facilitate host colonization. In recent years, the massive sequencing of many of them, such as the late blight of the potato Phytophthora infestans, has brought to light the diversity and the very large number of effectors (several hundred per species). This finding suggests that Oomycetes have the ability to set up different invasion strategies when in contact with a host. In addition, it is assumed that some of these effectors participate in the molecular dialogue that can be established between microorganisms in the environment close to the roots of the host (Rhizosphere). For a very large majority of these effectors, for which no analysis can predict their actual function, their implications in plant-microorganism and / or microbe-microbe interactions still remain to be determined.
Why are we interested in the effectors of the soil-borne oomycete Aphanomyces euteiches?
Devastating plant and animal parasites are found in the Aphanomyces genus of oomycete.
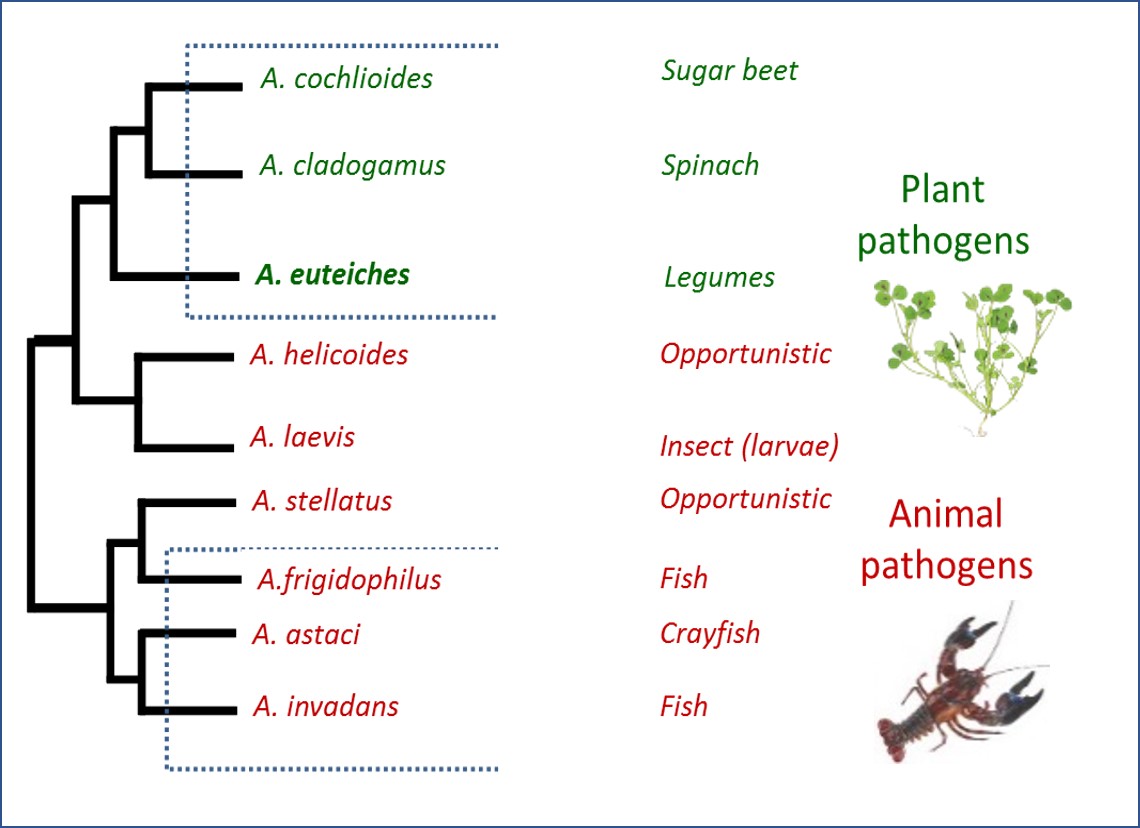
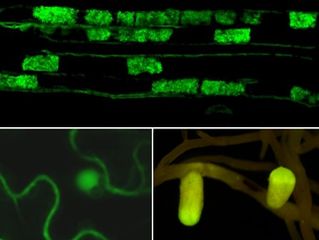
We are interested by Aphanomyces euteiches, a soil-borne pathogen of many legumes such as pea or alfalfa that limit the cultivation of these plants throughout the world. Since no chemical treatment or fully resistant legumes are available against Aphanomyces root rot, crop rotations are recommanded to limit the disease. A. euteiches also interacts with the model legume Medicago truncatula, which makes it possible to develop analyses that can include different types of symbiotic and pathogenic soil partners. Analysis of the genome of A. euteiches, available in the AphanoDB database (http://www.polebio.lrsv.ups-tlse.fr/aphano/) revealed to us a large repertoire of effectors with particularities specific to the genus Aphanomyces. A. euteiches genome is characterized by the absence of RxLR effectors, the presence of a large number of Crinkler effectors (CRNs) and of multiple Small Secreted Proteins (SSP).
Numerous CRNs and SSPs are detected in the nucleus of host cells, suggesting that affecting the genomic integrity of the host may be a novel mode of action for oomycetes. Our recent work has revealed the ability of a CRN effector to cause DNA damage, and of an SSP effector to cause nucleolar stress in the plant host. Having set up FRET-FLIM imaging techniques to characterize DNA / protein interactions in vivo, we are now focusing on specifying the mode of action of effectors interacting with nucleic acids both in plants (Microbial Effectors) and in microorganisms / microorganisms interactions (Ecological Effectors).

Participants: Laurent CAMBORDE (IE, PhD CNRS), Bernard DUMAS (DR, CNRS), Elodie GAULIN (MC, UTIII) and Students
Collaborations: BioPlantProducts
Efficiency of mycorrhizal symbiosis and competitiveness
Arbuscular mycorrhizal (AM) fungi are known as plant mutualists able to interact with an extremely large spectrum of hosts. The AM fungus model Rhizophagus irregularis, for instance, can virtually colonize any plant species that can establish AM symbiosis (estimated to 71% of land plant species). On the other side, each plant can be colonized simultaneously by tenth of different genotypes. AM fungi compete with each other to colonize plants, and host genotype-AM fungal genotype associations are selected based on the mutual nutritional rewards obtained by both partners. As a consequence, AM fungi have host preference, leading to more or less marked benefits for the plant.
We hypothesize that this symbiotic efficiency is higher when the couple plant/fungus are originating from the same location. It is this notion that we have explored through the study of diversity of AM fungal species and strains (of Rhizophagus irregularis) associated with maize and wheat plants, collected in domestication and non-endemic areas (respectively Mexico and Iran). After the studies on infectivity and symbiotic efficiency, the concept of competitiveness is being decrypted through the development of tools permitting strain quantification into co-inoculated roots.
Participants: Guillaume BECARD (PR UTIII), Francis CARBONNE (AI CNRS), Arthur MAES (PhD), Christophe ROUX (PR UTIII), Nathalie SEJALON-DELMAS (MC UTIII), Margot TRINQUIER (PhD student UTIII) and Students
Collaborations: Benoit LEFEBVRE (LIPME, France), Sébastien ROY (Agronutrition, France)
Genomics of Arbuscular Mycorrhizal (AM) Fungi
Associated to the previous works, we investigate the genomic signatures that support symbiotic efficiency for AM fungi. We focus on R. irregularis as this species is ubiquitous and present in annual crop plants. By comparing genomes of 6 strains of R. irregularis, we previously identified an unexpected intraspecific genome plasticity, with a pangenome estimated to 150 000 genes. Based on a new version of genome assembly and a specific pipeline for expressome analysis, we investigate transcriptomic signatures that could explain variations in the symbiotic efficiency of different R. irregularis strains according to different host plants.
Participants: Christophe ROUX (PR UTIII) and Students
Collaborations: Nicolas CORRADI (Un. Ottawa, Canada), Pierre Emmanuel COURTY (INRAE, Dijon), Mohamed ZOUINE (LRSV), BioPlantProducts
Symbiotic efficiency of Rhizobia & root development
Several soil bacteria collectively known as rhizobia can trigger the formation of symbiosis-dedicated organs in legumes: the root nodules, in which nitrogen fixation takes place. While a great deal of knowledge about symbiotic signaling and nitrogen fixation is now available, little is known about the tight and reciprocal links that exist between the maintenance of nodule development and an efficient interaction. Plant and bacterial factors are indeed required for the robust development of symbiotic nodules in legumes, which in turn, is necessary for the nitrogen fixation process, and reciprocally. Using Bradyrhizobium and Sinorhizobium model systems, we aim at identifying new symbiotic signals and mechanisms that are needed for the maintenance and the efficiency of rhizobial symbiosis. In addition, using the Medicago model system, we are characterizing how basic mechanisms of root development are used to rewire plant cells for AM and root nodule endosymbioses. In this frame, we are using single-cell approaches to decipher the transcriptome reprogramming of newly formed lateral roots, root nodules and AM-infected cells in Medicago and soybean (collab. S Bensmihen, LIPME and B Guillotin, NYU).
To reflect more natural and complex environments, we have established experimental systems and initiated approaches using diverse Legume crops (lupine, soybean, garden pea) to understand the effect of complex microbe-microbe interactions on symbiotic efficiency (collab. B Gourion, LIPME), with the ultimate goal to tackle these questions at the field scale.
Participants: Guillaume BECARD (PR UTIII), Jean-Malo COUZIGOU (CR CNRS), Jing ZHOU (PhD Student, UTIII, CSC grant) and Students
Collaborations: Sandra BENSMIHEN, Benjamin GOURION (LIPME, Toulouse, France), Bruno GUILLOTIN (NYU, USA).
Synergy of AM fungi and soil bacteria to improve AM symbiosis efficiency
Whereas AM fungi are known for their role in providing phosphorus to plant (P), the ability of extraradical mycelium to recruit organic and insoluble forms of mineral P in soil is limited.
AM fungi were described to be associated with bacteria in their vicinity – forming a microbial hyphosphere – that solubilize and mineralize organic and insoluble P. We study the association of R. irregularis with soil bacteria involved in P acquisition.
Participants: Guillaume BECARD (PR UTIII), Christophe ROUX (PR UTIII), Benoit DANILO (UTIII-Region Occitanie) and Students
Collaborations: BioPlantProducts
Endophytes
Endophyte fungi are fungi that can live into plant tissues. To the contrary of AM fungi which are necessarily endophytic and mutualists, endophytes which belong to Mucoro- Asco- and Basidiomycota are not always strictly endophytes, nor beneficial. However, thanks to the Next Generation Sequencing, more and more studies describe their diversity and suggest a link between this diversity and some stress resistance (drought and heavy metals, for example). After this step of description, the aim now is to decipher the relationships between endophytes and AM fungi hosted in the same root systems, as well as the cultural conditions that could promote the endophyte diversity and its ecological services.
Participants: Francis CARBONNE (AI CNRS), Arthur MAES (PhD), Nathalie SEJALON-DELMAS (MC, UTIII) and Students
Impact of selected microorganisms of rhizospheric microbiota
Root microbiota is an extraordinary complex biotic environment where microorganisms are confronted to one another and shaped by the composition of root exudate. In turn, microbiota plays a tremendous role in role helping plant to acquire nutrients and to fight against pathogenic microorganism.

Among the millions of microbial species which are present in root microbiota, some species can plays particular role by regulating the composition of microbiota, avoiding the development of pathogenic species, favouring the interaction of rots with symbiotic partners or stimulating plant development. In this research theme, conducting mostly in the frame of the Joint laboratory BioPlantproducts in collaboration with industrial partners (DE SANGOSSE and Agronutrition) we investigate molecular mechanisms underlying the activity of selected strains, an oomycete, Pythium oligandrum and bacterial strains belonging to the Streptomyces genus.
Incidence of pathogenic oomycetes on rhizosphere microbiota
Despite their low proportion in the soil, oomycetes can have dramatic effect in plant growth and probably on the composition of rhizosphere microbiota. By focusing on two oomycetes, Aphanomyces euteiches, a legume pathogen, and Pythium oligandrum, a mycoparasite, we investigate the effect of soilborne oomycetes on the establishment of pathogenic and symbiotic interactions and more broadly on the composition of the root microbiota.
 Pythium oligandrum is a ubiquitous soil-dwelling oomycete displaying strong antifungal and anti-oomycete activities. P oligandrum is also able to stimulate plant growth and interact with plant roots without causing disease symptoms. Our objective is to investigate how P. oligandrum interacts with pathogenic and symbiotic fungi and influences their interaction with plant roots.
Pythium oligandrum is a ubiquitous soil-dwelling oomycete displaying strong antifungal and anti-oomycete activities. P oligandrum is also able to stimulate plant growth and interact with plant roots without causing disease symptoms. Our objective is to investigate how P. oligandrum interacts with pathogenic and symbiotic fungi and influences their interaction with plant roots.
Incidence of Streptomycetes on rhizosphere microbiota
Streptomycetes are ubiquitous soil filamentous bacteria producing several antibiotic compounds among which fungicides and bactericides, and also able to produce some phytohormones.

The identification in their genome of original gene clusters involved in antibiotic synthesis raises the question of the incidence of these soil bacteria on our microbial models, and more generally on the root microbiota. For this project we used Streptomyces strains selected following a screening strategy to identify bacteria able to induce the plant immune system and to produce antifungal metabolites. Genetic and metabolomics tools are developed to identify specialized metabolites produced by streptomycetes in the rhizosphere and how their production is regulated. Importantly, the impact of streptomycete in the root microbiota and the role of their secondary metabolites are assessed notably on Medicago roots interacting with pathogens (A. euteiches) or symbiotic microorganisms (AM fungi, rhizobia).
Participants: Maryam HASHEMI HOSSEINABADI (PhD student, Marie-Curie), Clément NICOLLE (PhD, Student) Laurent CAMBORDE (IE, PhD CNRS), Kévin ADAM (IR), Bernard DUMAS (DR, CNRS), Elodie GAULIN (MC, UTIII) and Students
Collaborations: BioPlantProducts
Publications
View all our publications by clicking on this link here
Root associated Streptomyces produce galbonolides to modulate plant immunity and promote rhizosphere colonizationNicolle, C., Gayrard, D., Noël, A., Hortala, M., Amiel, A., Grat-Simeone, S., Le Ru, A., Marti, G., Pernodet, J-L., Lautru, S., Dumas, B. and Rey, T (2024) . ISME J doi: 10.1093/ismejo/wrae112
Deciphering transcriptomic and metabolomic wood responses to grapevine trunk diseases-associated fungi. Romeo-Olivan A, Chervin J, Breton C, Puech-Pages V, Fournier S, Marti G, Rodrigues O, Daydé J, Dumas B, Jacques A (2024) Phytofrontiers 10.1094/PHYTOFR-10-23-0132-R
2023
Complementary peptides represent a credible alternative to agrochemicals by activating translation of targeted proteins
Mélanie Ormancey, Bruno Guillotin, Rémy Merret, Laurent Camborde, Carine Duboé, Bertrand Fabre, Cécile Pouzet, Francis Impens, Delphi van Haver, Marie-Christine Carpentier, Hélène San Clemente, Marielle Aguilar, Dominique Lauressergues, Lars B Scharff, Carole Pichereaux, Odile Burlet-Schiltz, Cécile Bousquet-Antonelli, Kris Gevaert, Patrice Thuleau, Serge Plaza, Jean-Philippe Combier
Nature Communications, 2023, 14 (1), pp.254. ⟨10.1038/s41467-023-35951-0⟩
DOI : 10.1038/s41467-023-35951-0
https://hal.science/hal-03949768/file/Ormancey_2023.pdf BibTex
Rapid detection and quantification of Aphanomyces cochlioides in sugar beet
Valentina Rossi, Julia Bengtsson, Andrei Kiselev, Elodie Gaulin, Louise Holmquist, Laura J Grenville-Briggs
Journal of Plant Pathology, 2023, 105 (4), pp.1581-1591. ⟨10.1007/s42161-023-01490-2⟩
DOI : 10.1007/s42161-023-01490-2
https://ut3-toulouseinp.hal.science/hal-04428329/file/s42161-023-01490-2.pdf BibTex
The mycoparasite Pythium oligandrum induces legume pathogen resistance and shapes rhizosphere microbiota without impacting mutualistic interactions
Maryam Hashemi, Aurélien Amiel, Mohamed Zouaoui, Kévin Adam, Hélène San Clemente, Marielle Aguilar, Rémi Pendaries, Jean-Malo Couzigou, Guillaume Marti, Elodie Gaulin, Sébastien Roy, Thomas Rey, Bernard Dumas
Frontiers in Plant Science, 2023, 14, pp.1–17. ⟨10.3389/fpls.2023.1156733⟩
DOI : 10.3389/fpls.2023.1156733
https://hal.science/hal-04251678/file/Hashemi_fpls-14-1156733.pdf BibTex
Genome Sequence of the Streptomyces Strain AgN23 Revealed Expansion and Acquisition of Gene Repertoires Potentially Involved in Biocontrol Activity and Rhizosphere Colonization
Damien D. Gayrard, Clément Nicolle, Marine Veyssière, Kévin Adam, Yves Martinez, Céline Vandecasteele, Marie Vidal, Bernard Dumas, Thomas Rey
PhytoFrontiers, 2023, ⟨10.1094/PHYTOFR-11-22-0131-R⟩
DOI : 10.1094/PHYTOFR-11-22-0131-R
https://hal.science/hal-04244412/file/phytofr-11-22-0131-r.pdf BibTex
The root pathogen Aphanomyces euteiches secretes modular proteases in pea apoplast during host infection
Andrei Kiselev, Laurent Camborde, Laura Ossorio Carballo, Farnusch Kaschani, Markus Kaiser, Renier a L van der Hoorn, Elodie Gaulin
Frontiers in Plant Science, 2023, 14, ⟨10.3389/fpls.2023.1140101⟩
DOI : 10.3389/fpls.2023.1140101
https://hal.science/hal-04251844/file/fpls-14-1140101.pdf BibTex
Ontogenetic changes in root traits and root-associated fungal community composition in a heteroblastic epiphytic bromeliad
Celine Leroy, Arthur Maes, Eliane Louisanna, Nathalie Séjalon-Delmas, Amandine Erktan, Heidy Schimann
Oikos, 2023, 2023 (1), pp.e09213. ⟨10.1111/oik.09213⟩
DOI : 10.1111/oik.09213
https://hal.inrae.fr/hal-03625721/file/Leroy_etal_Oikos_2022.pdf BibTex
2022
Journal articles
Pathogenicity of animal and plant parasitic Aphanomyces spp and their economic impact on aquaculture and agriculture
Thomas Becking, Andrei Kiselev, Valentina Rossi, David Street-Jones, Frédéric Grandjean, Elodie Gaulin
Fungal Biology Reviews, 2022, 40: special issue on Oomycete-Plant and Animal Interactions, pp.1-18. ⟨10.1016/j.fbr.2021.08.001⟩
DOI : 10.1016/j.fbr.2021.08.001
https://ut3-toulouseinp.hal.science/hal-03416455/file/Pathogenicity%20of%20animal%20and%20plant%20parasitic%20Aphanomyces%20spp.pdf BibTex
Comparative Transcriptomics Suggests Early Modifications by Vintec® in Grapevine Trunk of Hormonal Signaling and Secondary Metabolism Biosynthesis in Response to Phaeomoniella chlamydospora and Phaeoacremonium minimum
Ana Romeo-Oliván, Justine Chervin, Coralie Breton, Thierry Lagravère, Jean Daydé, Bernard Dumas, Alban Jacques
Frontiers in Microbiology, 2022, 13, pp.898356. ⟨10.3389/fmicb.2022.898356⟩
DOI : 10.3389/fmicb.2022.898356
https://ut3-toulouseinp.hal.science/hal-03687473/file/Romeo-Oliv%C3%A1n_2022.pdf BibTex
Modification of Early Response of Vitis vinifera to Pathogens Relating to Esca Disease and Biocontrol Agent Vintec® Revealed By Untargeted Metabolomics on Woody Tissues
Justine Chervin, Ana Romeo-Oliván, Sylvie Fournier, Virginie Puech Pagès, Bernard Dumas, Alban Jacques, Guillaume Marti
Frontiers in Microbiology, 2022, 13, pp.835463. ⟨10.3389/fmicb.2022.835463⟩
DOI : 10.3389/fmicb.2022.835463
https://ut3-toulouseinp.hal.science/hal-03652523/file/Chervin_2022.pdf BibTex
Characterization of plant microRNA-encoded peptides (miPEPs) reveals molecular mechanisms from the translation to activity and specificity
Dominique Lauressergues, Mélanie Ormancey, Bruno Guillotin, Hélène San Clemente, Laurent Camborde, Carine Duboé, Sabine Tourneur, Pierre Charpentier, Amélie Barozet, Alain Jauneau, Aurélie Le Ru, Patrice Thuleau, Virginie Gervais, Serge Plaza, Jean-Philippe Combier
Cell Reports, 2022, 38 (6), pp.110339. ⟨10.1016/j.celrep.2022.110339⟩
DOI : 10.1016/j.celrep.2022.110339
https://ut3-toulouseinp.hal.science/hal-03602110/file/Lauressergues_2022.pdf BibTex
A Comprehensive Assessment of the Secretome Responsible for Host Adaptation of the Legume Root Pathogen Aphanomyces euteiches
Andrei Kiselev, Hélène San Clemente, Laurent Camborde, Bernard Dumas, Elodie Gaulin
Journal of Fungi, 2022, 8 (1), pp.88. ⟨10.3390/jof8010088⟩
DOI : 10.3390/jof8010088
https://ut3-toulouseinp.hal.science/hal-03562048/file/Kiselev_2022.pdf BibTex
Ants mediate community composition of root‐associated fungi in an ant‐plant mutualism
Celine Leroy, Arthur Quymanh Maes, Eliane Louisanna, Jean-Francois Carrias, Régis Céréghino, Bruno Corbara, Nathalie Séjalon-Delmas
Biotropica, 2022, 54 (3), pp.645-655. ⟨10.1111/btp.13079⟩
DOI : 10.1111/btp.13079
BibTex
2021
Journal articles
Taxonomic, phylogenetic, and functional diversity of root‐associated fungi in bromeliads: effects of host identity, life forms, and nutritional modes
Celine Leroy, Arthur Quymanh Maes, Eliane Louisanna, Heidy Schimann, Nathalie Séjalon-Delmas
New Phytologist, 2021, 231 (3), pp.1195-1209. ⟨10.1111/nph.17288⟩
DOI : 10.1111/nph.17288
BibTex
Lipid exchanges drove the evolution of mutualism during plant terrestrialization
Mélanie K Rich, Nicolas Vigneron, Cyril Libourel, Jean Keller, Xue Li, Mohsen Hajheidhari, Guru V Radhakrishnan, Aurélie Le Ru, Seydina Issa Diop, Giacomo Potente, Elena Conti, Danny Duijsings, Aurélie Batut, Pauline Le Faouder, Kyoichi Kodama, Junko Kyozuka, Erika Sallet, Guillaume Bécard, Marta Rodriguez-Franco, Thomas Ott, Justine Bertrand-Michel, Giles Oldroyd, Péter Szövényi, Marcel Bucher, Pierre-Marc Delaux
Science, 2021, 372 (6544), pp.864-868. ⟨10.1126/science.abg0929⟩
DOI : 10.1126/science.abg0929
https://hal.science/hal-03233313/file/Rich%20et%20al%202021%20Merged%20Author%20file.pdf BibTex
Distinct genetic bases for plant root responses to lipo-chitooligosaccharide signal molecules from distinct microbial origins
Maxime Bonhomme, Sandra Bensmihen, Olivier André, Emilie Amblard, Magali Garcia, Fabienne Maillet, Virginie Puech Pagès, Clare Gough, Sebastien Fort, Sylvain Cottaz, Guillaume Bécard, Christophe Jacquet
Journal of Experimental Botany, 2021, 72 (10), pp.3821-3834. ⟨10.1093/jxb/erab096⟩
DOI : 10.1093/jxb/erab096
https://hal.science/hal-02949578/file/2021_M_Bonhomme_HAL.pdf BibTex
The genome of Geosiphon pyriformis reveals ancestral traits linked to the emergence of the arbuscular mycorrhizal symbiosis
Mathu Malar C, Manuela Krüger, Claudia Krüger, Yan Wang, Jason E Stajich, Jean Keller, Eric C H Chen, Gokalp Yildirir, Matthew Villeneuve-Laroche, Christophe Roux, Pierre-Marc Delaux, Nicolas Corradi
Current Biology – CB, 2021, 31 (7), pp.1570-1577.e4. ⟨10.1016/j.cub.2021.01.058⟩
DOI : 10.1016/j.cub.2021.01.058
https://hal.science/hal-03327905/file/Malar_et_al_Geosiphon.pdf BibTex
2020
Vergnes S, Gayrard D, Veyssière M, Toulotte J, Martinez Y, Dumont V, Bouchez O, Rey T, Dumas B (2020) Phyllosphere colonisation by a soil Streptomyces promotes plant defense responses against fungal infection Mol Plant Microbe Interact 33:223-234
DOI: 10.1094/MPMI-05-19-0142-R.
Faure C, Veyssière M, Boëlle B, San Clemente H, Bouchez O, Lopez-Roques C, Chaubet A, Martinez Y, Bezouška K, Suchánek M, Gaulin E, Rey T, Dumas B. (2020) Long-Read genome sequence of the sugar beet rhizosphere mycoparasite Pythium oligandrum. G3: Genes, Genomes, Genetics
DOI: 10.1534/g3.119.40074
Petit E, Berger M, Camborde L, Vallejo V, Daydé J, Jacques A. (2020) Development of screening methods for functional characterization of UGTs from Stevia rebaudiana. Sci Rep 15;10(1):15137
DOI: 10.1038/s41598-020-71746-9
Fraisier-Vannier O, Chervin J, Cabanac G, Puech-Pages V, Fournier S, Durand V, Amiel A, Andre O, Benamar OA, Tsugawa H, Dumas B, Marti G. (2020) MS-CleanR: A feature-filtering workflow for untargeted LC-MS based metabolomics. Anal. Chem. 92:9971-9981
DOI :10.1021/acs.analchem.0c01594
Rush, TA, Puech-Pagès, V, Bascaules, A, Jargeat, P, Maillet, F, Haouy, A, Maës AQ, Carriel CC, Khokhani D, Keller-Pearson M, Tannous J, Cope KR, Garcia K, Maeda J, Johnson C, Kleven B, Choudhury QJ, Labbé J, Swift C, O’Malley MA, Bok JW, Cottaz S, Fort S, Poinsot V, Sussman MR, Lefort C, Nett J, Keller NP, Bécard, G, Ané, JM (2020) Lipo-chitooligosaccharides as regulatory signals of fungal growth and development. Nature communications, 11(1), 1-10.
DOI : 10.1038/s41467-020-17615-5.
Reinhardt, D, Roux, C, Corradi, N, Di Pietro, A (2020). Lineage-specific genes and cryptic sex: parallels and differences between arbuscular mycorrhizal fungi and fungal pathogens. Trends in Plant Science 26(2):111-123.
DOI : 10.1016/j.tplants.2020.09.006
Pons, S, Fournier, S, Chervin, C, Bécard, G, Rochange, S, Frei Dit Frey, N, Puech Pagès, V (2020). Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plos One 15(10), e0240886.
DOI : org/10.1371/journal.pone.0240886
2019
Camborde L, Raynaud C, Dumas B, Gaulin E. (2019) DNA-damaging effectors: new players in the effector arena. Trends Plant Sci. 24:1094-1101
DOI: 10.1016/j.tplants.2019.09.012.
Escouboué M, Camborde L, Jauneau A, Gaulin E, Deslandes L (2019) Preparation of plant material for analysis of protein-nucleic acid Interactions by FRET-FLIM. Methods Mol Biol 1991:69-77.
DOI: 10.1007/978-1-4939-9458-8_8.
Chervin J, Cabanac G, Audonnet M, Esquerré-Tugayé MT, Camborde L, Dumas B, Roux C, Talou T (2019) Deciphering the phylogeny of violets based on multiplexed genetic and metabolomic approaches Phytochemistry 163:99-110
DOI: 1016/j.phytochem.2019.04.001
Bonhomme M, Fariello MI, Navier H, Hajri A, Badis Y, Miteul H, Samac DA, Dumas B, Baranger A, Jacquet C, Pilet-Nayel ML (2019). A local score approach improves GWAS resolution and detects minor QTL: application to Medicago truncatula quantitative disease resistance to multiple Aphanomyces euteiches Heredity 123: 517–531
DOI: 10.1038/s41437-019-0235-x
Rey T, André O, Nars A, Dumas B, Gough C, Bottin A, Jacquet C. (2019) Lipo-chitooligosaccharide signalling blocks a rapid pathogen-induced ROS burst without impeding immunity. New Phytol. 221: 743-749
DOI: 10.1111/nph.15574
Leroy C, QuyManh Maes A, Louisanna E, Sejalon-Delmas N (2019) How significant are endophytic fungi in bromeliad seeds and seedlings? Effects on germination, survival and performance of two epiphytic plant species. Fungal Ecology 39:296-306.
DOI: 10.1016/j.funeco.2019.01.004
Pellegrin C, Daguerre Y, Ruytinx J, Guinet F, Kemppainen M, Frei dit Frey N, Puech-Pagès V, Hecker A, Pardo AG, Martin FM, Veneault-Fourrey C (2019). Laccaria bicolor MiSSP8 is a small-secreted protein decisive for the establishment of the ectomycorrhizal symbiosis. Environ Microbiol 21(10):3765-3779
DOI: 10.1111/1462-2920.14727
Calabrese, S, Cusant, L, Sarazin, A, Niehl, A, Erban, A, Brulé, D, Recorbet G, Wipf D, Roux C, Kopka J, Boller T, Courty, PE (2019). Imbalanced regulation of fungal nutrient transports according to phosphate availability in a symbiocosm formed by poplar, sorghum, and Rhizophagus irregularis. Frontiers in Plant Science, 10, 1617.
DOI: 10.3389/fpls.2019.01617
Cope KR, Bascaules A, Irving TB, Venkateshwaran M, Maeda J, Garcia K, Rush TA, Mad C, Labbé J, Jawdy S, Steigerwald E, Setzke J, Fung E, Schnell KG, Wang Y, Schlief N, Bücking H, Strauss StH, Maillet F, Jargeat P, Bécard G, Puech-Pagès V, Ané JM (2019) The ectomycorrhizal fungus Laccaria bicolor produces lipochitooligosaccharides and uses the common symbiosis pathway to colonize Populus. Plant Cell, 31 (10), 2386–2410
DOI: 10.1105/tpc.18.00676
Le Marquer M, Bécard G, Frei dit Frey N (2019) Arbuscular mycorrhizal fungi possess a CLAVATA3/Endosperm surrounding region-related gene that positively regulates symbiosis. New Phytol 222:1030-1042
DOI: 10.1111/nph.15643.
Mathé, C, Fawal, N, Roux, C, Dunand, C (2019). In silico definition of new ligninolytic peroxidase sub-classes in fungi and putative relation to fungal life style. Scientific Reports 9(1), 1-14.
DOI : 10.1038/s41598-019-56774-4
Morin E, Miyauchi S, San Clemente H, Chen ECH, Pelin A, de la Providencia I, Ndikumana S, Beaudet D, Hainaut M, Drula E, Kuo A, Tang N, Roy S, Viala J, Henrissat B, Grigoriev IV, Corradi N*, Roux C*, Martin F* (2019) Comparative genomics of Rhizophagus irregularis, R. cerebriforme, R. diaphanus and Gigaspora rosea highlights specific genetic features in Glomeromycotina. New Phytol 222: 1584–1598
DOI: 10.1111/nph.15687
Le Marquer M, San Clemente H, Roux C, Savelli B, Frei Dit Frey N (2019) Identification of new signalling peptides through a genome-wide survey of 250 fungal secretomes. BMC Genomics. 20(1):64.
DOI: 10.1186/s12864-018-5414-2
2018
Gaulin E, Pel JCM, Camborde L, San Clemente H, Courbier S, Dupouy MA, Lengelle J, Vayssiere M, Le Ru A, Grandjean F, Cordaux R, Moumen B, Gilbert C, Cano L, Aury JM, Guy J, Wincker P, Bouchez O, Klopp C, Dumas B (2018) Genomics analysis of Aphanomyces spp. identifies a new class of oomycete effector associated with host adaptation. BMC Biol 16:43
DOI: 10.1186/s12915-018-0508-5
Rey T, Jacquet C (2018) Symbiosis genes for immunity and vice versa. Curr Opin Plant Biol 44:64-71
DOI: 10.1016/j.pbi.2018.02.010.
Magne K, Couzigou JM, Schiessl K, Liu S, George J, Zhukov V, Sahl L, Boyer F, Iantcheva A, Mysore KS, Wen J, Citerne S, Oldroyd GED and Ratet P (2018) MtNODULE ROOT1 and MtNODULE ROOT2 are essential for Medicago truncatula indeterminate nodule identity. Plant Physiol 178(1):295-316
DOI: 10.1104/pp.18.00610
Mathieu S, Cusant L, Roux C, Corradi N (2018) Arbuscular mycorrhizal fungi: intraspecific diversity and pangenomes. New Phytol 220(4): 1129-1134
DOI: 10.1111/nph.15275
Pierart, A, Maes, AQ, Dumat, C, Sejalon-Delmas, N (2018). Vermicompost addition influences symbiotic fungi communities associated with leek cultivated in metal-rich soils. Environmental Science and Pollution Research, 26(20), 20040-20051.
DOI: 10.1007/s11356-018-2803-7
Pierart A, Dumat C, Maes AQ, Séjalon-Delmas N (2018) Influence of arbuscular mycorrhizal fungi on antimony phyto-uptake and compartmentation in vegetables cultivated in urban gardens. Chemosphere, 191:272-279
DOI: 10.1016/j.chemosphere.2017.10.058
Pierart A, Dumat C, Maes AQ, Roux C, Séjalon-Delmas N (2018) Opportunities and risks of biofertilization for leek production in urban areas: Influence on both fungal diversity and human bioaccessibility of inorganic pollutants. Sci Total Environ, 624:1140-1151
DOI: 10.1016/j.scitotenv.2017.12.100
Chen E, Morin E, Beaudet D, Noel J, Yildirir G, Ndikumana S, Charron P, Stonge C, Giorgi J, Kruger M, Marton T, Ropars J, Grigoriex IV, Hainaut M, Henrissat B, Roux C, Martin F & Corradi N (2018) High intraspecific genome diversity in the model arbuscular mycorrhizal symbiont Rhizophagus irregularis. New Phytol 220(4):1161-1171
DOI: 10.1111/nph.14989
2017
Rey T, Bonhomme M, Chatterjee A, Gavrin A, Toulotte J, Yang W, André O, Jacquet C, Schornack S (2017) The Medicago truncatula GRAS protein RAD1 supports arbuscular mycorrhiza symbiosis and Phytophthora palmivora susceptibility. (2017) J Exp Bot 68:5871-5881
DOI : doi.org/10.1093/jxb/erx398
Camborde L, Jauneau A, Brière C, Deslandes L, Dumas B, Gaulin E (2017) Detection of nucleic acid – protein interactions in plant leaves using fluorescence lifetime imaging microscopy. Nature Protocols 12:1933–1950
DOI : 10.1038/nprot.2017.076
Rey T, Dumas B. (2017) Plenty is not plague : Streptomyces symbiosis with crops. Trends Plant Sci 22:30-37
DOI: 10.1016/j.tplants.2016.10.008
Bécard G (2017) How plants communicate with their biotic environment, Adv Bot Res, 1st Edition, Academic Press 82, 404 pages
Gaulin E (2017) Effector-mediated communication of filamentous plant pathogens with their hosts. Adv Bot Res, 1st Edition, Academic Press 82:161-185
Rich M K, Courty PE, Roux C, Reinhardt D (2017) Role of the GRAS transcription factor ATA/RAM1 in the transcriptional reprogramming of arbuscular mycorrhiza in Petunia hybrida. BMC Genomics, 18(1), 589
Bécard G, Puech-Pagès V (2017) Communication entre les partenaires dans la symbiose mycorhizienne à arbuscules, in « Les sols et la vie souterraine » – Des enjeux majeurs en agroécologie. J.-F. Briat & D. Job (Coord.), Quae, 328 pages
Bécard G, Guidi L, Alizon S, Angelier F, Bezin L, Bowler C, Courchamp F, Dutreuil S, Faure M, Hibner U, Jebbar M, Kaltz O, Leulier F, Loudet O, Simonet P, Tost J, Vergnolle N (2017) L’être vivant dans son environnement, in « Étonnant vivant – Découvertes et promesses du XXIe siècle », C. Jessus & T. Gaude (Eds.), CNRS Edition, 328 pages
Hérivaux A, de Bernonville TD, Roux C, Clastre M, Courdavault V, Gastebois A, … & Papon N (2017) The identification of phytohormone receptor homologs in early diverging fungi suggests a role for plant sensing in land colonization by fungi. mBio, 8(1), e01739-16
DOI: 10.1128/mBio.01739-16
Nadal M, Sawers R, Naseem S, Bassin B, Kulicke C, Sharman A, An G, An K, Ahern KR, Romag A, Brutnell TP, Gutjahr C, Geldner N, Roux C, Martinoia E, Konopka JB & Paszkowski U (2017) An N-acetylglucosamine transporter required for arbuscular mycorrhizal symbioses in rice and maize. Nature Plants 3, 17073.
DOI:10.1038/nplants.2017.73
Kamel L, Tang N, Malbreil M, San Clemente H, Le Marquer M, Roux C, Frei Dit Frey N (2017) The comparison of expressed candidate secreted proteins from two Arbuscular mycorrhizal fungi unravels common and specific molecular tools to invade different host plants. Front Plant Sci 7;8:124.
DOI: 10.3389/fpls.2017.00124
Couzigou JM, Lauressergues D, André O, Gutjahr C, Guillotin B, Bécard G, Combier JP (2017) Positive gene regulation by a natural protective miRNA enables arbuscular mycorrhizal symbiosis. Cell Host Microbe 14, S1931-3128(16)30490-5.
DOI: 10.1016/j.chom.2016.12.00
Leroy A, Jauneau Y, Martinez A, Cabin-Flaman D, Gibouin J, Orivel, N. Séjalon-Delmas. (2017). Exploring fungus-plant N transfer in an ant-plant-fungi tripartite interaction. Annals of Botany, 120 : 417-426
DOI: /10.1093/aob/mcx064
2016
Dufour MC, Magnin, N, Dumas B, Vergnes S, Corio-Costet MF (2016) High-throughput gene-expression quantification of grapevine defense responses in the field using Microfluidic Dynamic Arrays. BMC Genomics 17:957
DOI: 10.1186/s12864-016-3304-z
Rey T, Laporte P, Bonhomme M, Jardinaud MF, Huguet S, Balzergue S, Dumas B, Niebel A, Jacquet C (2016) MtNF-YA1, a central transcriptional regulator of symbiotic nodule development, is also a determinant of Medicago truncatula susceptibility toward a root pathogen. Front Plant Sci 7 : 1837
DOI : 10.3389/fpls.2016.01837
Burgarella C, Chantret N, Gay L, Prosperi JM, Bonhomme M, Tiffin P, Young ND, Ronfort J. (2016) Adaptation to climate through flowering phenology : a case study in Medicago truncatula. Mol Ecol 25:3397-415
DOI: 10.1111/mec.13683
Ramirez-Garces D, Camborde L, Pel MJ, Jauneau A, Martinez Y, Neant I, Leclerc C, Moreau M, Dumas B, Gaulin E. (2016) CRN13 candidate effectors from plant and animal eukaryotic pathogens are DNA-binding proteins which trigger host DNA damage response. New Phytol 210: 602-617.
DOI: 10.1111/nph.13774.
Planchais S, Camborde L, Jupin I (2016) Protocols for studying protein stability in an Arabidopsis protoplast transient expression system. Methods Mol Biol 1450:175-94.
DOI: 10.1007/978-1-4939-3759-2_14.
Guillotin B, Couzigou JM, Combier JP (2016) NIN is involved in the regulation of arbuscular mycorrhizal symbiosis. Front Plant Sci 7:1704.
DOI: 10.3389/fpls.2016.01704
Gough C, Bécard G (2016) Strigolactones and lipochitooligosaccharides as molecular communication signals in the arbuscular mycorrhizal symbiosis, in « Molecular Mycorrhizal Symbiosis », F. Martin (Ed.), Wiley-Blackwell, 576 pages
Thiéry O, Vasar M, Jairus T, Davison J, Roux C, Kivistik PA, Metspalu A, Milani L, Saks Ü, Moora M, Zobel M, Öpik M (2016) Sequence variation in nuclear ribosomal small subunit, internal transcribed spacer and large subunit regions of Rhizophagus irregularis and Gigaspora margarita is high and isolate‐dependent. Molecular Ecology, 25(12), 2816-2832
DOI: 10.1111/mec.13655
Delaux PM, Radhakrishnan GV, Jayaraman D, Cheema J, Malbreil M, Volkening JD, Sekimoto H, Nishiyama T, Melkonian M, Pokorny L, Rothfels CJ, Winter Sederoff H, Stevenson DW, Surek B, Zhang Y, Sussman MR, Dunand C, Morris RJ, Roux C, Wong GKS, Oldroyd GED, Ané JM (2016) The algal ancestor of land plants was pre-adapted for symbiosis. PNAS, 112(43), 13390-13395
DOI: 10.1073/pnas.1515426112
Kamel L, Keller-Pearson M, Roux C, Ané JM (2016) Biology and evolution of arbuscular mycorrhizal symbiosis in the light of genomics. New Phytol 213 (2) 531-536
DOI: 10.1111/nph.14263
Guillotin B, Etemadi M, Audran C, Bouzayen M, Bécard G, Combier JP (2016) Sl-IAA27 regulates strigolactone biosynthesis and mycorrhization in tomato (var. MicroTom). New Phytol. 213 (3) 1124-1132
DOI: 10.1111/nph.14246
Couzigou JM, Combier JP (2016) Plant microRNAs : key regulators of root architecture and biotic interactions. New Phytol. 212 (1) 22-35
DOI: 10.1111/nph.14058
Couzigou JM, André O, Guillotin B, Alexandre M, Combier JP (2016) Use of microRNA-encoded peptide miPEP172c to stimulate nodulation in soybean. New Phytol 211(2) 379-81
DOI : 10.1111/nph.13991
Tang N, San Clemente H, Roy S, Bécard G, Zhao B, Roux C (2016) A survey of the gene repertoire of Gigaspora rosea unravels conserved features among Glomeromycota for obligate biotrophy. Front Microbiol 1 (7) 233
DOI : 10.3389/fmicb.2016.00233
2015
Martinez T, Texier H, Nahoum N, Lafitte C, Cioci G, Heux L, Dumas B, O’Donohue M, Gaulin E, Dumon C. (2015) Probing the functions of Carbohydrate Binding Modules in the CBEL protein from the Oomycete Phytophthora parasitica. PLoS One 10 : e0137481
Badis Y, Bonhomme M, Lafitte C, Huguet S, Balzergue S, Dumas B, Jacquet C (2015) Transcriptome analysis highlights preformed defenses and signaling pathways controlled by the prAe1 QTL, conferring partial resistance to Aphanomyces euteiches in Medicago truncatula. Mol. Plant.Pathol. 16 : 973-986
Bonhomme M, Boitard S, San Clemente H, Dumas B, Young N, Jacquet C (2015) Genomic signature of selective sweeps illuminates adaptation of Medicago truncatula to root-associated microorganisms. Mol. Biol. Evol. 2 : 2097–2110
Le Roux C, Huet G, Jauneau A, Camborde L, Tremousaygue D, Kraut A, Zhou B, Levaillant M, Adachi H, Yoshioka H, Raffaele S, Berthome R, Coute Y, Parker JE, Deslandes L (2015) A Receptor Pair with an Integrated Decoy Converts Pathogen Disabling of Transcription Factors to Immunity. Cell 161 : 1074-1088
Laffont C, Rey T, André O, Novero M, Kazmierczak T, Debellé F, Bonfante P, Jacquet C, Frugier F (2015) The CRE1 cytokinin pathway is differentially recruited depending on Medicago truncatula root environments and negatively regulates resistance to a pathogen. PLoS One. 10:e0116819
2014
Vergnes, S, Ladouce, N., Fournier, S., Ferhout, H., Attia, F., Dumas, B. (2014) Foliar treatments with Gaultheria procumbens essential oil induce defence responses and resistance against a fungal pathogen in Arabidopsis. Front. Plant Sci. 5:477
Bonhomme, M., André, O., Badis, Y., Ronfort, N., Burgarella, C., Chantret, N., Prosperi, J.M.,Briskine, R., Mudge, J., Debéllé, F., Navier, H., Miteul, H., Hajri, A., Baranger, A., Tiffin, P., Dumas, B., Pilet-Nayel, M.L., Young, N.D., Jacquet, C. (2014) High-density genome-wide association mapping identifies an F-box protein as the likely major component of Medicago truncatula resistance to Aphanomyces euteiches. New Phytol. 201:1328-42
2013
Nars A, Lafitte C, Chabaud M, Drouillard S, Mélida H, Danoun S, Le Costaouëc T, Rey T, Benedetti J, Bulone V, Barker DG, Bono JJ, Dumas B, Jacquet C, Heux L, Fliegmann J, Bottin A. (2013) Aphanomyces euteiches cell wall fractions containing novel glucan-chitosaccharides induce defense genes and nuclear calcium oscillations in the plant host Medicago truncatula. PLoS One 8:e75039
Larroque M, Belmas E, Martinez, M, Vergnes S, Ladouce N, Lafitte C, Gaulin E, Dumas B (2013) Pathogen-associated molecular pattern-triggered immunity and resistance to the root pathogen Phytophthora parasitica in Arabidopsis . J. Exp. Bot. 64 : 3615–3625
Gough C, Jacquet C (2013) Nod factor perception protein carries weight in biotic interactions. Trends Plant Sci. 18:566-574
Jiang RHY, de Bruijn I, Haas BJ, Belmonte R, Löbach L, Christie J, van den Ackerveken G, Bottin A, Bulone V, Dıaz-Moreno SM, Dumas B, Fan L, Gaulin E, Govers F, Grenville-Briggs LJ, Horner NR, Levin JZ, Mammella M, Meijer HJG, Morris P, Nusbaum C, Oome S, Phillips AJ, Rzeszutek E, van Rooyen D, Saraiva M, Secombes CJ, Seidl MF, Snel B, Stassen JHM, Sykes S, Tripathy S, van den Berg H, Vega-Arreguin JC, Wawra S, Young SK, Zeng Q, Dieguez-Uribeondo J, Russ C, Tyler BM, van West P (2013) Distinctive expansion of potential virulence genes in the genome of the oomycete fish pathogen Saprolegnia parasitica PLoS Genet. 9 : e1003272
Rey T, Nars A, Bonhomme M, Bottin A, Huguet S, Balzergue S, Jardinaud MF, Bono JJ, Cullimore J, Dumas B, Gough C, Jacquet C (2013) NFP, a LysM protein controlling Nod factor perception, also intervenes in Medicago truncatula resistance to pathogens. New Phytol. 198 : 875-886
Nars A, Rey T, Lafitte C, Vergnes S, Amatya S, Jacquet C, Dumas B, Thibaudeau C, Heux L, Bottin A, Fliegmann J (2013) An experimental system to study responses of Medicago truncatula roots to chitin oligomers of high degree of polymerization and other microbial elicitors. Plant Cell Rep. 32 : 489-502
2012
Larroque M, Barriot R, Bottin A, Barre A, Rouge P, Dumas B, Gaulin E (2012) The unique architecture and function of cellulose-interacting proteins in oomycetes revealed by genomic and structural analyses. BMC Genomics 13
2011
Jaulneau V, Lafitte C, Corio-Costet MF, Stadnik MJ, Salamagne S, Briand X, Esquerré-Tugayé, MT, Dumas B (2011) An Ulva armoricana extract protects plants against three powdery mildew pathogens Eur. J. Plant Pathol. 131 :393–401
Djébali N, Mhadhbi H, Lafitte C, Dumas B, Esquerré-Tugayé MT, Aouani ME and Jacquet C (2011) Hydrogen peroxide scavenging mechanisms are components of Medicago truncatula partial resistance to Aphanomyces euteiches Eur. J. Plant Pathol. 131 : 559-571
Larroque M, Ramirez D, Lafitte C, Borderies G, Dumas B, Gaulin E (2011) Expression and purification of a biologically active Phytophthora parasitica cellulose binding elicitor lectin in Pichia pastoris. Protein Expr Purif. 80 : 217-223
Baxter L, Tripathy S, Ishaque N, Boot N, Cabral A, Kemen E, Thines M, Ah-Fong A, Anderson R, Badejoko W, Bittner-Eddy P, Boore JL, Chibucos MC, Coates M, Dehal P, Delehaunty K, Dong S, Downton P, Dumas B, Fabro G, Fronick C, Fuerstenberg SI, Fulton L, Gaulin E, Govers F, Hughes L, Humphray S, Jiang RH, Judelson H, Kamoun S, Kyung K, Meijer H, Minx P, Morris P, Nelson J, Phuntumart V, Qutob D, Rehmany A, Rougon-Cardoso A, Ryden P, Torto-Alalibo T, Studholme D, Wang Y, Win J, Wood J, Clifton SW, Rogers J, Van den Ackerveken G, Jones JD, McDowell JM, Beynon J, Tyler BM (2010) Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330: 1549-1551
Jaulneau V, Cazaux C, Wong Sak Hoi J, Fournier S, Esquerré-Tugayé MT, Jacquet C, Dumas B (2010) Host and non-host resistance in Medicago–Colletotrichum interactions. Mol Plant-Microbe Interact 23: 1107–1117
Fammartino A, Verdaguer B, Fournier J, Tamietti G, Carbonne F, Esquerré-Tugayé MT, Cardinale F (2010) Coordinated transcriptional regulation of the divinyl ether biosynthetic genes in tobacco by signal molecules related to defense. Plant Physiol Biochem 48: 225-231
Gaulin E, Bottin A, Dumas B (2010) Sterol biosynthesis in oomycete pathogens. Plant Signal Behav 5: 258-260
Jaulneau V, Lafitte C, Jacquet C, Fournier S, Salamagne S, Briand X, Esquerré-Tugayé MT, Dumas B (2010) Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J Biomed Biotech 10: 525291
Levesque CA, Brouwer H, Cano L, Hamilton JP, Holt C, Huitema E, Raffaele S, Robideau GP, Thines M, Win J, Zerillo MM, Beakes GW, Boore JL, Busam D, Dumas B, Ferriera S, Fuerstenberg SI, Gachon CM, Gaulin E, Govers F, Grenville-Briggs L, Horner N, Hostetler J, Jiang RH, Johnson J, Krajaejun T, Lin H, Meijer HJ, Moore B, Morris P, Phuntmart V, Puiu D, Shetty J, Stajich JE, Tripathy S, Wawra S, van West P, Whitty BR, Coutinho PM, Henrissat B, Martin F, Thomas PD, Tyler BM, De Vries RP, Kamoun S, Yandell M, Tisserat N, Buell CR (2010) Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol 11: R73
Schornack S, van Damme M, Bozkurt TO, Cano LM, Smoker M, Thines M, Gaulin E, Kamoun S, Huitema E (2010) Ancient class of translocated oomycete effectors targets the host nucleus. Proc Natl Acad Sci USA 107: 17421-17426
Wong Sak Hoi J, Dumas B (2010) The STE12 and STE12-like proteins: fungal transcription factors regulating development and pathogenicity. Eukaryot Cell 9: 480-485
2009
Djebali N, Jauneau A, Ameline-Torregrosa C, Chardon F, Jaulneau V, Mathe C, Bottin A, Cazaux M, Pilet-Nayel ML, Baranger A, Aouani ME, Esquerré-Tugayé MT, Dumas B, Huguet T, Jacquet C (2009) Partial resistance of Medicago truncatula to Aphanomyces euteiches is associated with protection of the root stele and is controlled by a major QTL rich in proteasome-related genes. Mol Plant Microbe Interact 22: 1043-1055
Madoui MA, Bertrand-Michel J, Gaulin E, Dumas B (2009) Sterol metabolism in the oomycete Aphanomyces euteiches, a legume root pathogen. New Phytol 183: 291-300
Pilet-Nayel ML, Prosperi JM, Hamon C, Lesne A, Lecointe R, Le Goff I, Herve M, Deniot G, Delalande M, Huguet T, Jacquet C, Baranger A (2009) AER1, a major gene conferring resistance to Aphanomyces euteiches in Medicago truncatula. Phytopathology 99: 203-208
Tollot M, Wong Sak Hoi J, van Tuinen D, Arnould C, Chatagnier O, Dumas B, Gianinazzi-Pearson V, Seddas PM (2009) An STE12 gene identified in the mycorrhizal fungus Glomus intraradices restores infectivity of a hemibiotrophic plant pathogen. New Phytol 181: 693-707
2008
Ameline-Torregrosa C, Cazaux M, Danesh D, Chardon F, Cannon SB, Esquerre-Tugaye MT, Dumas B, Young ND, Samac DA, Huguet T, Jacquet C (2008) Genetic dissection of resistance to anthracnose and powdery mildew in Medicago truncatula. Mol Plant Microbe Interact 21: 61-69
Badreddine I, Lafitte C, Heux L, Skandalis N, Spanou Z, Martinez Y, Esquerré-Tugayé MT, Bulone V, Dumas B, Bottin A (2008) Cell wall chitosaccharides are essential components and exposed patterns of the phytopathogenic oomycete Aphanomyces euteiches. Eukaryot Cell 7: 1980-1993
Dumas B, Bottin A, Gaulin E, Esquerre-Tugaye MT (2008) Cellulose-binding domains: cellulose associated-defensive sensing partners? Trends Plant Sci 13: 160-164
Gaulin E, Madoui MA, Bottin A, Jacquet C, Mathé C, Couloux A, Wincker P, Dumas B (2008) Transcriptome of Aphanomyces euteiches: new oomycete putative pathogenicity factors and metabolic pathways. PLoS ONE 3: e1723
2007
Fammartino A, Cardinale F, Gobel C, Mene-Saffrane L, Fournier J, Feussner I, Esquerre-Tugaye MT (2007) Characterization of a divinyl ether biosynthetic pathway specifically associated with pathogenesis in tobacco. Plant Physiol 143: 378-388
Gaulin E, Haget N, Khatib M, Herbert C, Rickauer M, Bottin A (2007) Transgenic sequences are frequently lost in Phytophthora parasitica transformants without reversion of the transgene-induced silenced state. Can J Microbiol 53: 152-157
Gaulin E, Jacquet C, Bottin A, Dumas B (2007) Root rot disease of legumes caused by Aphanomyces euteiches. Mol Plant Pathol 8: 539-548
Madoui MA, Gaulin E, Mathé C, San Clemente H, Couloux A, Wincker P, Dumas B (2007) AphanoDB: a genomic resource for Aphanomyces pathogens. BMC Genomics 8: 471
Djebali N, Mhadhbi H, Jacquet C, Huguet T, Aouani ME (2007) Involvement of hydrogen peroxide, peroxidase and superoxide dismutase in response of Medicago truncatula lines differing in susceptibility to Phoma medicaginis infection. J. Phytopathol 155: 633-640
Wong Sak Hoi JW, Herbert C, Bacha N, O’Connell R, Lafitte C, Borderies G, Rossignol M, Rougé P, Dumas B (2007) Regulation and role of a STE12-like transcription factor from the plant pathogen Colletotrichum lindemuthianum. Mol Microbiol 64: 68-82
2006
Ameline-Torregrosa C, Dumas B, Krajinski F, Esquerre-Tugaye MT, Jacquet C (2006) Transcriptomic approaches to unravel plant-pathogen interactions in legumes. Euphytica 147 : 25-36
Gaulin E, Dramé N, Lafitte C, Torto-Alalibo T, Martinez Y, Ameline-Torregrosa C, Khatib M, Mazarguil H, Villalba-Mateos F, Kamoun S, Mazars C, Dumas B, Bottin A, Esquerré-Tugayé MT, Rickauer M (2006) Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen-associated molecular patterns. Plant Cell 18 : 1766-1777
2005
Boudart G, Jamet E, Rossignol M, Lafitte C, Borderies G, Jauneau A, Esquerré-Tugayé MT, Pont-Lezica R (2005) Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes : identification by mass spectrometry and bioinformatics. Proteomics 5 : 212-221
Colditz F, Braun HP, Jacquet C, Niehaus K, Krajinski F (2005) Proteomic profiling unravels insights into the molecular background underlying increased Aphanomyces euteiches-tolerance of Medicago truncatula. Plant Mol Biol 59 : 387-406
Prost I, Dhondt S, Rothe G, Vicente J, Rodriguez MJ, Kift N, Carbonne F, Griffiths G, Esquerre-Tugaye MT, Rosahl S, Castresana C, Hamberg M, Fournier J (2005) Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol 139 : 1902-1913
Siriputthaiwan P, Jauneau A, Herbert C, Garcin D, Dumas B (2005) Functional analysis of CLPT1, a Rab/GTPase required for protein secretion and pathogenesis in the plant fungal pathogen Colletotrichum lindemuthianum. J Cell Sci 118 : 323-329
2004
Cluzet S, Torregrosa C, Jacquet C, Lafitte C, Fournier J, Mercier L, Salamagne S, Briand X, Esquerré-Tugayé MT, Dumas B (2004) Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from the green algae Ulva spp. Plant Cell Environ 27 : 917-928
Herbert C, O’Connell R, Gaulin E, Salesses V, Esquerre-Tugaye MT, Dumas B (2004) Production of a cell wall-associated endopolygalacturonase by Colletotrichum lindemuthianum and pectin degradation during bean infection. Fungal Genet Biol 41 : 140-147
Khatib M, Lafitte C, Esquerré-Tugayé MT, Bottin A, Rickauer M (2004) The CBEL elicitor of Phytophthora parasitica var. nicotianae activates defence in Arabidopsis thaliana via three different signalling pathways. New Phytol. 162 : 501-510
O’Connell R, Herbert C, Sreenivasaprasad S, Khatib M, Esquerré-Tugayé MT, Dumas B (2004) A novel Arabidopsis–Colletotrichum pathosystem for the molecular dissection of plant-fungal interactions. Mol Plant Microbe Interact 17 : 272-282
Torregrosa C, Cluzet S, Fournier J, Huguet T, Gamas P, Prosperi JM, Esquerré-Tugayé MT, Dumas B, Jacquet C (2004) Cytological, genetic, and molecular analysis to characterize compatible and incompatible interactions between Medicago truncatula and Colletotrichum trifolii. Mol Plant Microbe Interact 17 : 909-920
2003
Borderies G, Jamet E, Lafitte C, Rossignol M, Jauneau A, Boudart G, Monsarrat B, Esquerré-Tugayé MT, Boudet A, Pont-Lezica R (2003) Proteomics of loosely bound cell wall proteins of Arabidopsis thaliana cell suspension cultures : a critical analysis. Electrophoresis 24 : 3421-3432
Boudart G, Charpentier M, Lafitte C, Martinez Y, Jauneau A, Gaulin E, Esquerré-Tugayé MT, Dumas B (2003) Elicitor activity of a fungal endopolygalacturonase in tobacco requires a functional catalytic site and cell wall localization. Plant Physiol 131 : 93-101
Mène-Saffrané L, Esquerré-Tugayé MT, Fournier J (2003) Constitutive expression of an inducible lipoxygenase in transgenic tobacco decreases susceptibility to Phytophthora parasitica var. nicotianae. Mol Breeding 12 : 271-282
Theses
Ongoing PhDs
- Clement NICOLLE (2020-2023) Rôle des Streptomyces racinaires dans le fonctionnement du microbiote racinaire (Allocation ministérielle)
- Malo BOURGET (2020-2023) Les associations plantes-symbiotes comme drivers des relations dans le fonctionnement biodiversité-écosystème (Labex TULIP SYNERSYS project
- Nina GAZAL (2019-2022) Développement d’un matériau de construction isolant à base de mycélium (Allocation ministérielle)
- Margot TRINQUIET (2020-2024). Effet des pratiques agronomiques sur le microbiote des racines de blé et sur l’efficacité symbiotique des champignons mycorhiziens à arbuscules (Université de Toulouse-Région Occitanie)
Former PhDs
- Andrei KISELEV (2022) Molecular mechanisms of the interaction of the oomycete Aphanomyces euteiches with its environment and its plant host
- Maryam HASHEMI (2022) Impact of mycoparasite and root endophyte Pythium oligandrum on plant root-microbe interactions
- Arthur MAES (2021) Etude bioinformatique de la dispersion, de l’infectivité et de la compétitivité des populations de champignons mutualistes (CIFRE)
- Laurent CAMBORDE (2020) Caractérisation fonctionnelle de différents effecteurs candidats de l’oomycète Aphanomyces euteiches, parasite racinaire des légumineuses Lire
- Charlène FAURE (2020) Mécanismes cytologiques et moléculaires du pouvoir pathogène de Pythium oligandrum, oomycète mycoparasite
- Damien GAYRARD(2020) Activités stimulatrices des défense de plantes et antifongique d’une souche de Streptomyces sp. utilisée en tant qu’agent de biocontrôle
- Loïc CUSANT (2019) La boîte à outil génétique des champignons mycorhiziens à arbuscules pour leurs échanges carbonés avec les plantes hôtes. (Allocation ministérielle)
- Laurent KAMEL (2018) . Caractérisation de protéines sécrétées du champignon Rhizophagus irregularis – Criblage de leur effet sur l’établissement de la symbiose endomycorhizienne. (CIFRE)
- Bruno GUILLOTIN (2018). Autorégulation de la symbiose mycorhizienne à arbuscules Lire
- Nianwu TANG (2017). Transcriptomics of Gigaspora rosea
- Thomas MARTINEZ (2015) Les oomycètes, microorganismes pathogènes de plantes : nouvelles ressources biotechnologiques pour l’exploitation de la biomasse ligno-cellulosique
- Yacine BADIS (2014) Déterminants génétiques de la résistance de Medicago truncatula à Aphanomyces euteiches
- Mohammad ETEMADI (2014) Implication de l’auxine dans la symbiose endomycorhizienne à arbuscule chez la tomtate
- Diana RAMIREZ-GARCES, (2014) Analyse des effecteurs Crinklers (CRN) de l’oomycète Aphanomyces euteiches. Lire
- Mathilde MALBREIL (2014) Le dialogue moléculaire pendant la symbiose endomycorhizienne : perception et émission de signaux symbiotiques par le champignon Glomus intraradices. Lire
- Olivier ANDRE (2014) Analyse génétique de la résistance quantitative de Medicago truncatula au parasite racinaire Aphanomyces euteiches
- Coline BALZERGUE (2013) Université Paul Sabatier, Toulouse III. Régulation de la symbiose endomycorhizienne par le partenaire végétal. Lire
- Johann LOUARN (2013) Université Paul Sabatier, Toulouse III. Exploitation de la symbiose endomycorhizienne pour l’amélioration de la résistance à l’Orobanche chez le tournesol.
- Amaury NARS (2013) Caractérisation structurale et perception par la plante hôte Medicago truncatula des chitosaccharides pariétaux d’Aphanomyces euteiches, parasite de légumineuses. Lire
- Thomas REY (2013) Mise en évidence et caractérisation d’interconnexions moléculaires entre symbiose et résistance chez la légumineuse modèle Medicago truncatula
- Damien FORMEY DE SAINT LOUVENT (2012) Analyse du génome de Glomus intraradices et étude de la régulation génique chez le champignon et la plante hôte lors de l’établissement de la symbiose endomycorhizienne. Lire
- Jérôme LAPARRE (2012) Dynamique métabolique de l’établissement de la symbiose obligatoire des champignons mycorhiziens à arbuscules. Lire
- Matthieu LARROQUE (2011) Mécanismes de surveillance de l’intégrité pariétale et rôle dans l’induction des défenses des plantes. Lire
- Pierre-Marc DELAUX (2011) Contenu en strigolactones du maïs. Recherche de nouvelles strigolactones chez les organismes photosynthétiques ancestraux. Lire
- Valérie JAULNEAU (2010) Mode d’action de nouvelles molécules stimulatrices des défenses des plantes. (Confidentielle)
- Marc CAZAUX (2009) Analyse génétique de la résistance de la légumineuse Medicago truncatula à Colletotrichum trifolii. Lire
- M.Amine MADOUI (2009) Génomique fonctionnelle de l’interaction Medicago truncatula/Aphanomyces euteiches. Lire
- Arnaud BESSERER (2008) Étude des mécanismes d’action des strigolactones sur les champignons endomycorhiziens à arbuscules. Lire
- Ilham BADREDDINE (2008) Etudes des surfaces cellulaires d’Aphanomyces euteiches parasite de légumineuses.Lire
- Nacer DJEBALI (2008) Etude des mécanismes de résistance de la plante modèle Medicago truncatula vis-à-vis de deux agents pathogènes majeurs des légumineuses cultivées : Phoma medicaginis et Aphanomyces euteiches. Lire
- Maria-Victoria GOMEZ-ROLDA (2008) Rôle des strigolactones dans la symbiose mycorhizienne à arbuscules. Lire
- Seyed Kazem SABBAGH (2008) Adaptation à la pénétration racinaire de deux Ustilaginaceae parasites du maïs : Ustilago maydis et Sporisorium reilianum – Analyse microscopique et transcriptomique. Lire
- Joanne WONG SAK HOI (2007). Analyse fonctionnelle du facteur de transcription CLSTE12, impliqué dans le pouvoir pathogène du champignon phytopathogène Colletotrichum lindemtuhianum.
- Piyawan SIRIPUTTHAIWAN (2005). Sécrétion de protéines et pouvoir pathogène des champignons parasites de plantes : rôle d’une Rab/GTPase impliquée dans le transport vésiculaire.
- Carine TORREGROSA (2004) Caractérisation cytologique, génétique et moléculaire de l’interaction Colletotrichum trifolii/Medicago truncatula.
- Moustafa KHATIB (2004) Expression par transgenèse d’un éliciteur de Phytophthora dans le Tabac et Arabidopsis et étude du phénotype.
- Sonja KOSUTA (2003) Des facteurs diffusibles, produits par les champignons mycorhiziens à arbuscules, induisent des réponses symbiotiques au niveau des racines de Medicago truncatula.
- Corentin HERBERT (2003) Analyse moléculaire de la régulation de l’expression des gènes codant les endopolygalacturonases de Colletotrichum lindemuthianum, agent de l’anthracnose du Haricot.
- Elodie GAULIN (2002) Etudes fonctionnelles de CBEL, éliciteur de Phytophthora parasitica nicotianae.
lrsv.gestion@univ-tlse3.fr
Phone
Standard : 05.34.32.38.01
Fax : 05.34.32.38.02
Find us
24, chemin de Borde-Rouge.
31320, Auzeville-Tolosane. FRANCE










